This is an old revision of this page, as edited by Neodop (talk | contribs) at 23:25, 27 May 2011. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 23:25, 27 May 2011 by Neodop (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)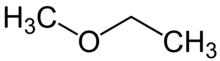
| |

| |
| Names | |
|---|---|
| IUPAC name Methoxyethane | |
| Other names
Methyl ethyl ether Ethyl methyl ether | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.128.000 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C3H8O |
| Molar mass | 60.096 g·mol |
| Appearance | Clear, colorless liquid |
| Melting point | −139 °C (−218 °F; 134 K) |
| Boiling point | 7.6 °C (45.7 °F; 280.8 K) |
| Viscosity | 0.224 cP at 25 °C |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Extremely Flammable (F+), Liquefied gas |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Methoxyethane, also known as ethyl methyl ether, is an ethyl group with a bonded methoxy. Methoxyethane is a colorless gaseous ether with a medicine-like odor. It is extremely flammable, and its inhalation may cause asphyxiation or dizzyness. As a Lewis base, it can react with Lewis acids to form salts and reacts violently with oxidizing agents.
References
| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Methoxyethane" – news · newspapers · books · scholar · JSTOR (May 2010) (Learn how and when to remove this message) |
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |