This is an old revision of this page, as edited by Pashihiko (talk | contribs) at 07:25, 9 June 2011. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 07:25, 9 June 2011 by Pashihiko (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
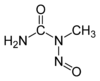
| IUPAC name 1-Methyl-1-nitrosourea | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Abbreviations | NMU | ||
| ECHA InfoCard | 100.010.618 | ||
| KEGG | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
SMILES
| |||
| Properties | |||
| Chemical formula | C2H5N3O2 | ||
| Molar mass | 103.08 g/mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
N-Nitroso-N-methylurea (NMU) is a highly reliable carcinogen, mutagen, and teratogen. NMU is an alkylating agent, and exhibits its toxicity by transferring its methyl group to nucleobases in nucleic acids.
NMU is the traditional precursor in the synthesis of diazomethane. However, since it is unstable at temperatures beyond 20 °C and shock-sensitive to a degree, for this purpose it has become obsolete and replaced by other (N-methyl)nitrosamides. Most chemical supply houses have stopped carrying it.
Acute exposure to NMU in humans can result in skin and eye irritation, headache, nausea, and vomiting. NMU is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals (IARC 1972, 1978, 1987). Various cancers induced in animal models include: squamous cell carcinomas of the forestomach, sarcomas and gliomas of the brain, adenocarcinomas of the pancreas, leukemia, and lymphomas. However, the actual potential for human exposure is quite limited, as the chemical is not produced or used in large quantities
NMU is teratogenic and embryotoxic, resulting in craniofacial (cleft palate) and skeletal defects, increased fetal resorption, and fetal growth retardation. Exposure to NMU during pre-implantation, post-implantation, organogenesis, or by paternal exposure can result in these effects.
References
- Hazardous Substance Fact Sheet for NMU New Jersey Department of Health and Senior Services
- ^ NMU Substance Profile NTP, Report on Carcinogens, Eleventh Edition
- Wada, A., et al. (1994). Induction of Congenital Malformations in Mice by Paternal Methylnitrosourea Treatment. Congenital Anomalies 34:65-70.
- Nagao, T., et al. (1991). Induction of Fetal Malformations After Treatment of Mouse Embryos with Methylnitrosourea at the Preimplantation Stages. Teratogenesis, Carcinogenesis, and Mutagenesis 11:1-10.
- Faustman, E., et al. (1989). In Vitro Developmental Toxicity of Five Direct-Acting Alkylating Agents in Rodent Embryos: Structure-Activity Patterns. Teratology 40:199-210.