This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 08:56, 10 June 2011 (Updating {{chembox}} (changes to verified and watched fields - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref') per Chem/Drugbox validation (report [[Wikip). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 08:56, 10 June 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (changes to verified and watched fields - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref') per Chem/Drugbox validation (report [[Wikip)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)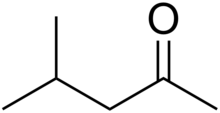
| |
| Names | |
|---|---|
| IUPAC name 4-Methylpentan-2-one | |
| Other names Isopropylacetone, Hexone, Isobutyl methyl ketone, 4-Methylpentan-2-one, 4-methyl-2-pentanone, 4-methylpentan-2-one, 2-methyl-4-pentanone, 2-methylpropyl methyl ketone, 4-methyl-2-oxopentane, MIK, isobutylmethyl ketone, MIBK, isohexanone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.003.228 |
| KEGG | |
| RTECS number |
|
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C6H12O |
| Molar mass | 100.16 g/mol |
| Appearance | colorless liquid |
| Density | 0.802 g/mL, liquid |
| Melting point | −84.7 °C (−120.5 °F; 188.5 K) |
| Solubility in water | 1.91 g/100 mL (20 °C) |
| Refractive index (nD) | 1.3958 |
| Viscosity | 0.58 cP at 20.0 °C |
| Structure | |
| Dipole moment | 4.2 D |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Flash point | 14 °C |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Methyl isobutyl ketone (MIBK) is the organic compound with the formula (CH3)2CHCH2C(O)CH3. This colourless liquid, a ketone, is widely used as a solvent.
Production
Methyl isobutyl ketone is manufactured from acetone via a three-step process. Firstly acetone undergoes an aldol condensation to give diacetone alcohol, which readily dehydrates to give mesityl oxide. Mesityl oxide can then be hydrogenated to give MIBK:
Modern processes combine these three steps into one.
Several million kilograms are produced annually.
Uses
MIBK is used as a solvent for nitrocellulose, lacquers, and certain polymers and resins.
Precursor to 6PPD
Another major use is as a precursor to N-(1,3-dimethylbutyl)-N'-phenyl-p-phenylene diamine (6PPD), an antiozonant used in tires. 6PPD is prepared by reductive coupling of MIBK with 4-aminodiphenylamine.
Solvent and niche applications
Unlike the other common ketone solvents, acetone and MEK, MIBK has quite low solubility in water, making it useful for liquid-liquid extraction. It has a similar polarity to ethyl acetate, but greater stability towards aqueous acid and base. It can be used to extract gold, silver and other precious metals from cyanide solutions, such as those found at gold mines, to determine the levels of those dissolved metals. Diisobutyl ketone (DIBK), a related lipophilic ketone, is also used for this purpose. Methyl isobutyl ketone is also used as a denaturing agent for denatured alcohol. When mixed with water or isopropyl alcohol MIBK serves as a developer for PMMA electron beam lithography resist. MIBK is used as a solvent for CS in the preparation of the CS spray used currently by British police forces.
References
- , Uhde Technology Profile: MIBK
- ^ Stylianos Sifniades, Alan B. Levy, “Acetone” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- Peter J Gray; Stark, MM; Gray, P. J; Jones, G R. N (2000). "CS gas is not CS spray - formulation is important" (Response to editorial). BMJ. 321 (7252): 26. doi:10.1136/bmj.321.7252.46. PMC 1127688. PMID 10939811.
External links
- International Chemical Safety Card 0511
- National Pollutant Inventory - Methyl isobutyl ketone fact sheet
- NIOSH Pocket Guide to Chemical Hazards