This is an old revision of this page, as edited by John (talk | contribs) at 02:48, 14 June 2011 (→Synthesis and reactions: ce). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 02:48, 14 June 2011 by John (talk | contribs) (→Synthesis and reactions: ce)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)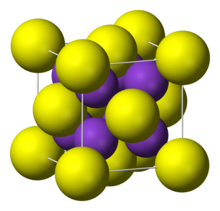
| |
| Names | |
|---|---|
| IUPAC name Potassium sulfide | |
| Other names
Dipotassium monosulfide, Dipotassium sulfide, Potassium monosulfide, Potassium sulphide | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.013.816 |
| RTECS number |
|
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | K2S |
| Molar mass | 110.262 g/mol |
| Appearance | pure: colourless impure: yellow-brown |
| Density | 1.8 g/cm |
| Melting point | 840 °C |
| Boiling point | decomposes |
| Solubility in water | converts to KSH, KOH |
| Solubility in other solvents | soluble in ethanol and glycerol |
| Structure | |
| Crystal structure | antiFluorite |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Dangerous for the environment (N) |
| Related compounds | |
| Other cations | Sodium sulfide, Iron(II) sulfide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Potassium sulfide is the inorganic compound with the formula K2S. The colourless solid is rarely encountered, because it reacts readily with water, a reaction that affords potassium bisulfide (KSH) and potassium hydroxide (KOH).
Structure
It adopts "antifluorite structure," which means that the small K ions occupy the tetrahedral (F) sites in fluorite, and the larger S centers occupy the eight-coordinate sites. Li2S, Na2S, and Rb2S crystallize similarly.
Synthesis and reactions
It can be produced by heating K2SO4 with carbon (coke):
- K2SO4 + 4 C → K2S + 4 CO
In the laboratory, a number of methods exist. K2S arises from the reaction of potassium and sulfur. In the laboratory, this synthesis is usually conducted by combining a solution of potassium in anhydrous ammonia with elemental sulfur. Another method of making K2S in laboratory involves the reaction of potassium permanganate and elemental sulfur:
- 2 KMnO4 + S → K2S + 2 MnO2 + 2 O2
Sulfide is highly basic, consequently K2S completely and irreversibly hydrolyzes in water according to the following equation:
- K2S + H2O → KOH + KSH
For many purposes, this reaction is inconsequential since the mixture of SH and OH behaves as a source of S. Other alkali metal sulfides behave similarly.
Use in fireworks
Potassium sulfides are formed when black powder is burned and are important intermediates in many pyrotechnic effects, such as senko hanabi and some glitter formulations.
See also
References
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 200.
- Shimizu, Takeo. "Fireworks: the Art, Science, and Technique." Pyrotechnica Publications: Austin, 1981. ISBN 0-929388-05-4.
| Potassium compounds | |
|---|---|
| H, (pseudo)halogens | |
| chalcogens | |
| pnictogens | |
| B, C group | |
| transition metals | |
| organic | |