This is an old revision of this page, as edited by MartinBotIII (talk | contribs) at 16:44, 23 July 2011 (fix MSDS link (ilo.org) using AWB). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 16:44, 23 July 2011 by MartinBotIII (talk | contribs) (fix MSDS link (ilo.org) using AWB)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |
| |
| Names | |
|---|---|
| IUPAC name Lead(II) oxide | |
| Other names
Lead monoxide Litharge Massicot Plumbous oxide | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.013.880 |
| RTECS number |
|
| UN number | 3288 |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | PbO |
| Molar mass | 223.20 g/mol |
| Appearance | red or yellow powder |
| Density | 9.64 g/cm |
| Melting point | 888 °C (1,630 °F; 1,161 K) |
| Boiling point | 1,477 °C (2,691 °F; 1,750 K) |
| Solubility in water | insoluble |
| Solubility | insoluble in dilute alkalis soluble in concentrated alkalis soluble in HCl |
| Structure | |
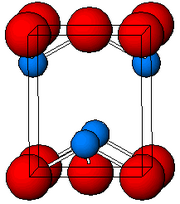
| Crystal structure | tetragonal, tP4 |
| Space group | P4/nmm, No. 129 |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Lead sulfide Lead selenide Lead telluride |
| Other cations | Carbon monoxide Silicon monoxide Tin(II) oxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Lead(II) oxide is the inorganic compound with the formula PbO. Lead(II) oxide occurs in two polymorphs, red, having a tetragonal crystal structure and yellow, having an orthorhombic crystal structure. Both forms occur naturally as rare minerals: the red form is known as litharge and the yellow form is known as massicot.
Preparation and structure
PbO may be prepared by heating lead metal in air at approx. 600 °C. At this temperature it is also the end product of oxidation of other lead oxides in air:
- PbO2 –(293 °C)→ Pb12O19 –(351 °C)→ Pb12O17 –(375 °C)→ Pb3O4 –(605 °C)→ PbO
Thermal decomposition of lead(II) nitrate or lead carbonate also results in the PbO formation:
- 2 Pb(NO3)2 → 2 PbO + 4 NO2 + O2
- PbCO3 → PbO + CO2
As determined by X-ray crystallography, the compound features pyramidal four-coordinate Pb center. The pyramidal nature indicates the presence of a stereo-chemically active lone pair of electrons.
Reactions
The red and yellow forms of this material are related by a small change in enthalpy: PbO(red) → PbO(yellow) ΔH = 1.6 kJ/mol
PbO is amphoteric, which means that it reacts with both acids and with bases. With acids, it forms salts of Pb via the intermediacy of oxo clusters such as . With strong base, PbO dissolves to form plumbite(II) salts: PbO + H2O + OH →
Applications
PbO is produced on a large scale as an intermediate in the conversion of lead ores, mainly galena into metallic lead. The consumption of lead, and hence the processing of PbO, correlates with the number of automobiles because it remains the key component of automotive lead-acid batteries.
PbO is used extensively in manufacturing of lead glasses and ceramic glazes as well as in fine dinnerware. For such applications, the PbO is converted in situ to lead silicate, which is less toxic. Other less dominating applications include the vulcanization of rubber and the production of certain pigments and paints. PbO is used in cathode ray tube glass to block X-ray emission, but mainly in the neck and funnel because it can cause discoloration when used in the faceplate. Strontium oxide is preferred for the faceplate.
Niche or declining uses
A mixture of PbO with glycerine sets to a hard, waterproof cement that has been used to join the flat glass sides and bottoms of aquaria, and was also once used to seal glass panels in window frames. It is a component of lead paints.
PbO is used in certain condensation reactions in organic synthesis.
Health issues
Main article: Lead poisoning
Lead oxide may be fatal if swallowed or inhaled. It causes irritation to skin, eyes, and respiratory tract. It affects gum tissue, central nervous system, kidneys, blood, and reproductive system. It can bioaccumulate in plants and in mammals.
References
- Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- N.N. Greenwood, A. Earnshaw, "Chemistry of Elements", 2nd edition, Butterworth-Heinemann, 1997.
- Wells, A. F. (1984), Structural Inorganic Chemistry (5th ed.), Oxford: Clarendon Press, ISBN 0-19-855370-6
- Charles A. Sutherland, Edward F. Milner, Robert C. Kerby, Herbert Teindl, Albert Melin, Hermann M. Bolt “Lead” in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a15_193.pub2
- Dodd S. Carr "Lead Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinhiem. doi:10.1002/14356007.a15_249
- Corson, B. B. (1936). "1,4-Diphenylbutadiene". Organic Syntheses. 16: 28; Collected Volumes, vol. 2, p. 229.
- "Lead (II) oxide". International Occupational Safety and Health Information Centre. Retrieved 2009-06-06.
External links
- Case Studies in Environmental Medicine - Lead Toxicity
- ToxFAQs: Lead
- National Pollutant Inventory - Lead and Lead Compounds Fact Sheet
- Webelements PbO
| Lead compounds | |
|---|---|
| Pb(II) | |
| Pb(II,IV) | |
| Pb(IV) | |