This is an old revision of this page, as edited by Rjwilmsi (talk | contribs) at 21:29, 23 July 2011 (→Titanium-carbon clusters: Journal cites:, added 1 issue number, using AWB (7751)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 21:29, 23 July 2011 by Rjwilmsi (talk | contribs) (→Titanium-carbon clusters: Journal cites:, added 1 issue number, using AWB (7751))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)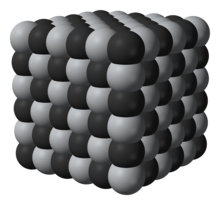
| |
| Identifiers | |
|---|---|
| CAS Number | |
| ECHA InfoCard | 100.031.916 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | TiC |
| Molar mass | 59.89 g/mol |
| Appearance | black powder |
| Density | 4.93 g/cm |
| Melting point | 3,160 °C (5,720 °F; 3,430 K) |
| Boiling point | 4,820 °C (8,710 °F; 5,090 K) |
| Solubility in water | insoluble in water |
| Structure | |
| Crystal structure | Cubic, cF8 |
| Space group | Fm3m, No. 225 |
| Coordination geometry | Octahedral |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Titanium carbide, TiC, is an extremely hard (Mohs 9-9.5) refractory ceramic material, similar to tungsten carbide.
It is commercially used in tool bits. It has the appearance of black powder with NaCl-type face centered cubic crystal structure. It is mainly used in preparation of cermets, which are frequently used to machine steel materials at high cutting speed.
The resistance to wear, corrosion, and oxidation of a tungsten carbide-cobalt material can be increased by adding 6-30% of titanium carbide to tungsten carbide. This forms a solid solution that is more brittle and susceptible to breakage than the original material.
Tool bits without tungsten content can be made of titanium carbide in nickel-cobalt matrix cermet, enhancing the cutting speed, precision, and smoothness of the workpiece. This material is sometimes called high-tech ceramics and is used as a heat shield for atmospheric reentry of spacecraft. The substance may be also polished and used in scratch-proof watches.
It can be etched with reactive-ion etching.
The mineralogical form is very rare and called khamrabaevite - (Ti,V,Fe)C.
Titanium-carbon clusters
A surprisingly stable cluster with formula Ti8C12, was detected in 1992. The 20 atoms were conjectured to be arranged as the vertices of a dodecahedron, with the titanium atoms at the corners of a cube However, this claim was soon disputed by Linus Pauling who proposed an alternative arrangement — with the Ti atoms still at the corners of a cube, but with the carbon atoms pushed inwards so as to be nearly coplanar with the faces of that cube.
References
- ^ Guo, Bc; Kerns, Kp; Castleman, Aw, Jr (1992). "Ti8C12+-Metallo-Carbohedrenes: A New Class of Molecular Clusters?". Science. 255 (5050): 1411–1413. doi:10.1126/science.255.5050.1411. ISSN 0036-8075. PMID 17801229.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Guo, Bc; Wei, S; Purnell, J; Buzza, S; Castleman, Aw, Jr (1992). "Metallo-Carbohedrenes M8C12+ (M = V, Zr, Hf, and Ti): A Class of Stable Molecular Cluster Ions". Science. 256 (5056): 515–516. doi:10.1126/science.256.5056.515. ISSN 0036-8075. PMID 17787948.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - L Pauling (1992). "Molecular structure of Ti8C12 and related complexes". Proc. Natl. Acad. Sci. USA. 89 (17): 8175. doi:10.1073/pnas.89.17.8175. PMID 11607323.
| Titanium compounds | |||||
|---|---|---|---|---|---|
| Titanium(II) |
| ||||
| Titanium(III) |
| ||||
| Titanium(IV) |
| ||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |