This is an old revision of this page, as edited by Makecat (talk | contribs) at 03:14, 5 August 2011. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 03:14, 5 August 2011 by Makecat (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)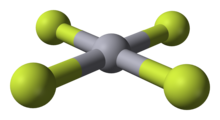
| |

| |
| Properties | |
|---|---|
| Chemical formula | HgF4 |
| Molar mass | 276.58 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Mercury(IV) fluoride, HgF4, is the first mercury compound to be discovered with the metal in the oxidation state IV. Mercury, like the other group 12 elements (cadmium and zinc), has an sd electron configuration and generally only forms bonds involving its s orbital. This means that the highest oxidation state mercury normally attains is II, and for this reason it is generally considered a post-transition metal instead of a transition metal.
History
Speculation about higher oxidation states for mercury had been around since the 1970s, and theoretical calculations in the 1990s predicted that it should be stable in the gas phase, with a square-planar geometry consistent with a formal d configuration. However, experimental proof remained elusive until 2007, when HgF4 was first prepared using solid neon and argon for matrix isolation at a temperature of 4 K. The compound was detected using infrared spectroscopy. Analysis of density functional theory and coupled cluster calculations showed that the d orbitals are involved in bonding, leading to the suggestion that mercury should be considered a transition metal after all. However, that conclusion has been challenged by W. B. Jensen with the argument that HgF4 only exists under highly atypical nonequilibrium conditions and should best be considered as an exception.
Explanation
Theoretical studies suggest that mercury is unique among the natural elements of group 12 in forming a tetrafluoride, and attribute this observation to relativistic effects. According to calculations, the tetrafluorides of the "less relativistic" elements cadmium and zinc are unstable and eliminate a fluorine molecule, F2, to form the metal-difluoride complex. On the other hand, the tetrafluoride of the "more relativistic" synthetic element 112, copernicium, is predicted to be more stable.
References
- "High Oxidation States: Mercury tetrafluoride synthesized".
- "Elusive Hg(IV) species has been synthesized under cryogenic conditions". 2007-10-12.
- Xuefang Wang (2007). "Mercury Is a Transition Metal: The First Experimental Evidence for HgF4". Angew. Chem. Int. Ed. 46 (44): 8371–8375. doi:10.1002/anie.200703710. PMID 17899620.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - William B. Jensen (2008). "Is Mercury Now a Transition Element?". J. Chem. Educ. 85: 1182–1183. doi:10.1021/ed085p1182.
| Mercury compounds | |||
|---|---|---|---|
| Mercury(I) | |||
| Mercury(II) |
| ||
| Mercury(IV) |
| ||
| Amalgams | |||
| Mercury cations | |||