This is an old revision of this page, as edited by Beetstra (talk | contribs) at 11:12, 9 August 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'UNII').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 11:12, 9 August 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'UNII').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article is an orphan, as no other articles link to it. Please introduce links to this page from related articles; try the Find link tool for suggestions. (April 2011) |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.034.939 |
| Chemical and physical data | |
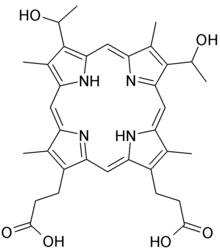
| Formula | C34H38N4O6 |
| Molar mass | 598.69 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Hematoporphyrin (Photodyn, Sensibion) is an endogenous porphyrin formed by the acid hydrolysis of hemoglobin. Nencki and Zaleski determined its chemical structure in 1900.
Hematoporphyrin has been used as an antidepressant and antipsychotic since the 1920s.
See also
References
- "SpringerLink - Journal Article".
- O'Neil, Maryadele J. (2001). The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Rahway, NJ: Merck Research Laboratories. ISBN 0-911910-13-1.
- "HEMATOPORPHYRIN AS A THERAPEUTIC AGENT IN THE PSYCHOSES -- Strecker et al. 90 (6): 1157 -- Am J Psychiatry".
| Antipsychotics (N05A) | |
|---|---|
| Typical |
|
| Disputed |
|
| Atypical |
|
| Others |
|
| |
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |