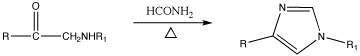This is an old revision of this page, as edited by Beetstra (talk | contribs) at 13:24, 9 August 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:24, 9 August 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)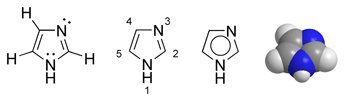
| |
| Names | |
|---|---|
| IUPAC name 1H-Imidazole | |
| Other names
1,3-diazole glyoxaline (archaic) 1,3-diazacyclopenta-2,4-diene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.473 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H4N2 |
| Molar mass | 68.077 g/mol |
| Appearance | white or pale yellow solid |
| Density | 1.23 g/cm, solid |
| Melting point | 89-91 °C (362-364 K) |
| Boiling point | 256 °C (529 K) |
| Solubility in water | miscible |
| Acidity (pKa) | 14.5 |
| Basicity (pKb) | 6.993 |
| Structure | |
| Crystal structure | monoclinic |
| Coordination geometry | planar 5-membered ring |
| Dipole moment | 3.61D |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Corrosive |
| Flash point | 146 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents. This ring system is present in important biological building blocks, such as histidine, and the related hormone histamine. Imidazole can serve as a base and as a weak acid. Many drugs contain an imidazole ring, such as antifungal drugs and nitroimidazole.
Discovery
Imidazole was first synthesized in 1858, but various imidazole derivatives had been discovered as early as the 1840s. His synthesis, as shown below, used glyoxal and formaldehyde in ammonia to form imidazole (or glyoxaline, as it was originally named). This synthesis, while producing relatively low yields, is still used for creating C-substituted imidazoles.
In one microwave modification, the reactants are benzil, benzaldehyde and ammonia in glacial acetic acid, forming 2,4,5-triphenylimidazole (Lophine).
Structure and properties
Imidazole is a 5-membered planar ring, which is soluble in water and other polar solvents. It exists in two equivalent tautomeric forms, 1H-imidazole and 3H-imidazole, because the hydrogen atom can be located on either of the two nitrogen atoms. Imidazole is a highly polar compound, as evidenced by a calculated dipole of 3.61D, and is entirely soluble in water. The compound is classified as aromatic due to the presence of a sextet of π-electrons, consisting of a pair of electrons from the protonated nitrogen atom and one from each of the remaining four atoms of the ring.
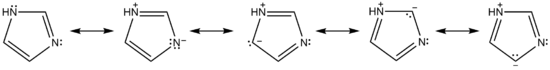
Some resonance structures of imidazole are shown below:
Amphotericity
Imidazole is amphoteric. That is, it can function as both an acid and as a base. As an acid, the pKa of imidazole is 14.5, making it less acidic than carboxylic acids, phenols, and imides, but slightly more acidic than alcohols. The acidic proton is located on N-1. As a base, the pKa of the conjugate acid (cited above as pKBH to avoid confusion between the two) is approximately 7, making imidazole approximately sixty times more basic than pyridine. The basic site is N-3.
Preparation

Imidazole can be synthesized by numerous methods besides the Debus method. Many of these syntheses can also be applied to different substituted imidazoles and imidazole derivatives simply by varying the functional groups on the reactants. In literature, these methods are commonly categorized by which and how many bonds form to make the imidazole rings. For example, the Debus method forms the (1,2), (3,4), and (1,5) bonds in imidazole, using each reactant as a fragment of the ring, and thus this method would be a three-bond-forming synthesis. A small sampling of these methods is presented below.
- Formation of one bond
The (1,5) or (3,4) bond can be formed by the reaction of an imidate and an α-aminoaldehyde or α-aminoacetal, resulting in the cyclization of an amidine to imidazole. The example below applies to imidazole when R=R1=Hydrogen.
- Formation of two bonds
The (1,2) and (2,3) bonds can be formed by treating a 1,2-diaminoalkane, at high temperatures, with an alcohol, aldehyde, or carboxylic acid. A dehydrogenating catalyst, such as platinum on alumina, is required.
The (1,2) and (3,4) bonds can also be formed from N-substituted α-aminoketones and formamide with heat. The product will be a 1,4-disubstituted imidazole, but here since R=R1=Hydrogen, imidazole itself is the product. The yield of this reaction is moderate, but it seems to be the most effective method of making the 1,4 substitution.
- Formation of four bonds
This is a general method that is able to give good yields for substituted imidazoles. In essence, it is an adaptation of the Debus method called the Debus-Radziszewski imidazole synthesis. The starting materials are substituted glyoxal, aldehyde, amine, and ammonia or an ammonium salt.
- Formation from other heterocycles
Imidazole can be synthesized by the photolysis of 1-vinyltetrazole. This reaction will give substantial yields only if the 1-vinyltetrazole is made efficiently from an organotin compound, such as 2-tributylstannyltetrazole. The reaction, shown below, produces imidazole when R=R1=R2=Hydrogen.
Imidazole can also be formed in a vapor-phase reaction. The reaction occurs with formamide, ethylenediamine, and hydrogen over platinum on alumina, and it must take place between 340 and 480°C. This forms a very pure imidazole product.
Biological significance and applications
Imidazole is incorporated into many important biological molecules. The most pervasive is the amino acid histidine, which has an imidazole side-chain. Histidine is present in many proteins and enzymes and plays a vital part in the structure and binding functions of hemoglobin. Histidine can be decarboxylated to histamine, which is also a common biological compound. It is a component of the toxin that causes urticaria, which is another name for allergic hives. The relationship between histidine and histamine are shown below:

One of the applications of imidazole is in the purification of His-tagged proteins in immobilised metal affinity chromatography(IMAC). Imidazole is used to elute tagged proteins bound to Ni ions attached to the surface of beads in the chromatography column. An excess of imidazole is passed through the column, which displaces the His-tag from nickel co-ordination, freeing the His-tagged proteins.
Imidazole has become an important part of many pharmaceuticals. Synthetic imidazoles are present in many fungicides and antifungal, antiprotozoal, and antihypertensive medications. Imidazole is part of the theophylline molecule, found in tea leaves and coffee beans, that stimulates the central nervous system. It is present in the anticancer medication mercaptopurine, which combats leukemia by interfering with DNA activities.
Pharmaceutical derivatives
The substituted imidazole derivatives are valuable in treatment of many systemic fungal infections. Imidazoles belong to the class of Azole antifungals, which includes ketoconazole, miconazole, and clotrimazole.
For comparison, another group of azoles is the triazoles, which includes fluconazole, itraconazole, and voriconazole . The difference between the imidazoles and the triazoles involves the mechanism of inhibition of the cytochrome P450 enzyme. The N3 of the imidazole compound binds to the heme iron atom of ferric cytochrome P450, whereas the N4 of the triazoles bind to the heme group. The triazoles have been shown to have a higher specificity for the cytochrome P450 than imidazoles, thereby making them more potent than the imidazoles.
Industrial applications
Imidazole has been used extensively as a corrosion inhibitor on certain transition metals, such as copper. Preventing copper corrosion is important, especially in aqueous systems, where the conductivity of the copper decreases due to corrosion.
Many compounds of industrial and technological importance contain imidazole derivatives. The thermostable polybenzimidazole PBI contains imidazole fused to a benzene ring and linked to a benzene, and acts as a fire retardant. Imidazole can also be found in various compounds that are used for photography and electronics.
Salts of imidazole
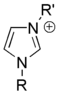
Salts of imidazole where the imidazole ring is in the cation are known as imidazolium salts (for example, imidazolium chloride). These salts are formed from the protonation or substitution at nitrogen of imidazole. These salts have been used as ionic liquids and precursors to stable carbenes. Salts where a deprotonated imidazole is an anion are also possible; these salts are known as imidazolide salts (for example, sodium imidazolide).
Related heterocycles
- Benzimidazole, an analog with a fused benzene ring
- Dihydroimidazole or benzimidazoline, an analog where 4,5-double bond is saturated
- Pyrrole, an analog with only one nitrogen atom in position 1
- Oxazole, an analog with the nitrogen atom in position 1 replaced by oxygen
- Thiazole, an analog with the nitrogen atom in position 1 replaced by sulfur
- Pyrazole, an analog with two adjacent nitrogen atoms
- Triazoles, analogs with three nitrogen atoms
References
- Alan R. Katritzky; Rees. Comprehensive Heterocyclic Chemistry. Vol. 5, p.469-498, (1984).
- Grimmett, M. Ross. Imidazole and Benzimidazole Synthesis. Academic Press, (1997).
- Brown, E.G. Ring Nitrogen and Key Biomolecules. Kluwer Academic Press, (1998).
- Pozharskii, A.F, et al. Heterocycles in Life and Society. John Wiley & Sons, (1997).
- Heterocyclic Chemistry TL Gilchrist, The Bath press 1985 ISBN 0-582-01421-2
- Heinrich Debus (1858). "Ueber die Einwirkung des Ammoniaks auf Glyoxal". Annalen der Chemie und Pharmacie. 107 (2): 199–208. doi:10.1002/jlac.18581070209.
- Microwave-Mediated Synthesis of Lophine: Developing a Mechanism To Explain a Product Crouch, R. David; Howard, Jessica L.; Zile, Jennifer L.; Barker, Kathryn H. J. Chem. Educ. 2006 83 1658
- US patent 6,177,575, A. J. Arduengo, "Process for Manufacture of Imidazoles", issued 2001-01-23
- Comprehensive Pharmacy Review, Leon Shargel, 6th edition, p930.
- Veterinary Pharmacology and Therapeutics, Riviere and Papich, 9th ed. p1019-1020.