This is an old revision of this page, as edited by Beetstra (talk | contribs) at 13:25, 9 August 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:25, 9 August 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |

| |
| Names | |
|---|---|
| IUPAC names
Correct new structure (upper pic.): 1,1'-methylenebis{3-urea} Erroneous old structure (lower pic.): 1,1'-methylenebis{3-urea} | |
| Other names
Imidurea, Germall 115;
N',N''-methylenebisurea]; 1-- 3- carbamoylamino]methyl]urea | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.049.411 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C11H16N8O8 |
| Molar mass | 388.29 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Imidazolidinyl urea" – news · newspapers · books · scholar · JSTOR (February 2010) (Learn how and when to remove this message) |
Imidazolidinyl urea is an antimicrobial preservative used in cosmetics . It is chemically related to diazolidinyl urea which is used in the same way. Imidazolidinyl urea acts as a formaldehyde releaser.
Safety
Some people have a contact allergy to imidazolidinyl urea causing dermatitis. Such people are often also allergic to diazolidinyl urea.
Chemistry
Imidazolidinyl urea was poorly characterized until recently and still has a wrong CAS structure assigned to it. New data show that the hydroxymethyl functional group of the imidazolidine ring is attached to the carbon, not the nitrogen atom :

|
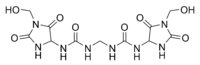
|
| Correct new structure | Erroneous old structure |
Synthesis
Imidazolidinyl urea is produced by the chemical reaction of allantoin and formaldehyde in the presence of sodium hydroxide solution and heat. The reaction mixture is then neutralized with hydrochloric acid and evaporated:
Commercial imidazolidinyl urea is a mixture of different formaldehyde addition products including polymers.
References
- Review of toxicological data (NTP NIEHS)
- ^
Lehmann SV, Hoeck U, Breinholdt J, Olsen CE, Kreilgaard B. (2006). "Characterization and chemistry of imidazolidinyl urea and diazolidinyl urea". Contact Dermatitis. 54 (1): 50–58. doi:10.1111/j.0105-1873.2006.00735.x. PMID 16426294.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
