This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 21:17, 9 August 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:W). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 21:17, 9 August 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:W)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)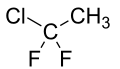
| |
| Names | |
|---|---|
| IUPAC name 1-Chloro-1,1-difluoroethane | |
| Other names Freon 142b | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.000.811 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2H3ClF2 |
| Molar mass | 100.49 g·mol |
| Appearance | Colorless gas |
| Melting point | −131 °C (−204 °F; 142 K) |
| Boiling point | −10 °C (14 °F; 263 K) |
| Solubility in water | Slight |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Asphyxiant |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
1-Chloro-1,1-difluoroethane, also known by trade names including Freon 142b is a haloalkane with the chemical formula CH3CClF2. It is primarily used as a refrigerant.
References
- "Safety data for 1-chloro-1,1-difluoroethane". Physical and Theoretical Chemistry Laboratory. University of Oxford. Retrieved 27 May 2011.