This is an old revision of this page, as edited by Smokefoot (talk | contribs) at 00:57, 12 August 2011 (trim procedural details (WP:NOTMANUAL)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 00:57, 12 August 2011 by Smokefoot (talk | contribs) (trim procedural details (WP:NOTMANUAL))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |
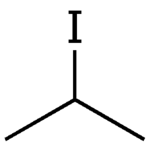
| |
| Names | |
|---|---|
| IUPAC name 2-iodopropane | |
| Other names iododimethylmethane, isopropyl iodide, 2-propyliodide, sec-propyl iodide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.000.782 |
| RTECS number |
|
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C3H7I |
| Molar mass | 169.99 |
| Appearance | Colourless liquid |
| Density | 1.703 |
| Melting point | −90.0 °C (−130.0 °F; 183.2 K) |
| Boiling point | 89.5 °C (193.1 °F; 362.6 K) |
| Solubility in water | 0.14 g/100 ml at 12.5 °C |
| Solubility in ethanol | fully miscible |
| Solubility in diethyl ether | fully miscible |
| Solubility in chloroform | fully miscible |
| Solubility in benzene | fully miscible |
| Refractive index (nD) | 1.4997 |
| Viscosity | 8.841 cP at 0 °C 6.971 cP at 20 °C |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Possible carcinogen. Harmful if swallowed, inhaled and in contact with skin. Eye, respiratory and mucous membrane irritant. |
| Flash point | 42 °C |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Isopropyl iodide is the organoiodine compound with the formula (CH3)2CHI. It is colorless, flammable, and volatile. Organic iodides are light-sensitive and take on a yellow colour upon storage, owing to the formation of iodine.
Preparation
Isopropyl iodide is prepared by iodination of isopropyl alcohol using hydrogen iodide or, equivalently, with a mixture of glycerol, iodine, and phosphorus. An alternative preparation involves the reaction of 2-propyl bromide with an acetone solution of potassium iodide (KI):
- (CH3)2CHBr + KI → (CH3)2CHI + KBr
References
- Merck Index of Chemicals and Drugs, 9th ed., monograph 5074
- Textbook of Practical Organic Chemistry, 5th Edition, Prentice Hall, 1989
This article about an organic halide is a stub. You can help Misplaced Pages by expanding it. |