This is an old revision of this page, as edited by BogBot (talk | contribs) at 00:58, 3 September 2011 (populated new fields in {{drugbox}} and reordered per bot approval. Report errors and suggestions to User_talk:BogBot). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 00:58, 3 September 2011 by BogBot (talk | contribs) (populated new fields in {{drugbox}} and reordered per bot approval. Report errors and suggestions to User_talk:BogBot)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound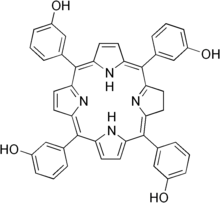 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| License data | |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.152.970 |
| Chemical and physical data | |
| Formula | C44H32N4O4 |
| Molar mass | 680.74 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
| (verify) | |
Temoporfin (INN) is a photosensitizer (based on porphyrin) used in photodynamic therapy for the treatment of squamous cell carcinoma of the head and neck . It is marketed in the European Union under the brand name Foscan. The US FDA deemed Foscan non-approvable in 2000. The EU approved its use in June 2001.
Good results were obtained in 21 of 35 patients treated in Germany.
It is photoactivated at 652 nm i.e. by red light.
Patients can remain photosensitive for several weeks after treatment.
Further reading
References
- Lorenz KJ, Maier H (2008). "". HNO (in German). 56 (4): 402–9. doi:10.1007/s00106-007-1573-1. PMID 17516041.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ O'Connor, Aisling E, Gallagher, William M, Byrne, Annette T (2009). "Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochemistry and Photobiology, Sep/Oct 2009". Photochemistry and Photobiology.
{{cite news}}: CS1 maint: multiple names: authors list (link) - http://www.highbeam.com/doc/1P2-18794532.html Foscan approval saves Scotia's skin.
- http://www.springerlink.com/content/g74w224824v8l013/
- "Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy". Photochemistry and Photobiology. 2009.
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |