This is an old revision of this page, as edited by Materialscientist (talk | contribs) at 13:05, 5 September 2011 (restore article). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:05, 5 September 2011 by Materialscientist (talk | contribs) (restore article)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)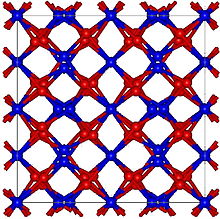
| |
| Names | |
|---|---|
| Other names Erbium oxide, erbia | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.031.847 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Er2O3 |
| Molar mass | 382.56 g/mol |
| Appearance | pink crystals |
| Density | 8.64 g/cm |
| Melting point | 2344°C |
| Boiling point | 3290°C |
| Structure | |
| Crystal structure | Cubic, cI80 |
| Space group | Ia-3, No. 206 |
| Thermochemistry | |
| Heat capacity (C) | 108.5 J·mol·K |
| Std molar entropy (S298) |
155.6 J·mol·K |
| Std enthalpy of formation (ΔfH298) |
-1897.9 kJ·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Erbium oxide, a pink solid, is a compound of erbium sometimes used as a colouring for glasses and a dopant for optical fibres and optical amplifiers. It was partially isolated by Carl Gustaf Mosander in 1843, and first obtained in pure form in 1905 by Georges Urbain and Charles James.
Erbium oxide can be also used as a burnable neutron poison for nuclear fuel.
It can react with acids to form the corresponding erbium(III) salts:
Er2O3 + 6 HCl → 2 ErCl3 + 3 H2O
References
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 4–57. ISBN 0849305942.
- Aaron John Ihde (1984). The development of modern chemistry. Courier Dover Publications. p. 378–379. ISBN 0486642356.
External links
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |
| Erbium compounds | |
|---|---|