This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 21:07, 6 September 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[WP:CHEMVALID|Chem/Drugbox validation). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 21:07, 6 September 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[WP:CHEMVALID|Chem/Drugbox validation)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)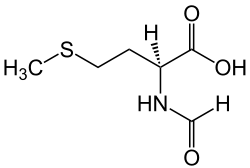
| |
| Names | |
|---|---|
| IUPAC name (S)-2-Formylamino-4-methylsulfanylbutanoic acid | |
| Other names
2-Formylamino-4-methylsulfanyl-butyric acid Formylmethionine N-Formyl(methyl)homocysteine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| EC Number |
|
| PubChem CID | |
SMILES
| |
| Properties | |
| Chemical formula | C6H11NO3S |
| Molar mass | 177.22 g/mol |
| Supplementary data page | |
| N-Formylmethionine (data page) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
N-Formylmethionine (fMet) is a proteinogenic amino acid found in Bacteria and related Prokaryotic organelles. It is a derivative of the amino acid methionine in which a formyl group has been added to the amino group. It is specifically used for initiation of protein synthesis, and may be removed after.
fMet plays a crucial part in the protein synthesis of bacteria, mitochondria and chloroplasts. It is not used in cytosolic protein synthesis of eukaryotes, where eukaryotic nuclear genes are translated. It is also not used by Archaea. In the human body, fMet is recognized by the immune system as foreign material and stimulates the body to fight against potential infection.
Function in protein synthesis
fMet is a starting residue in the synthesis of proteins in bacteria, and, consequently, is located at the N-terminus of the growing polypeptide. fMet is delivered to the ribosome (30S) - mRNA complex by a specialized tRNA (tRNA) which has a 3'-UAC-5' anticodon that is capable of binding with the 5'-AUG-3' start codon located on the mRNA.
fMet is coded by the same codon as methionine, AUG. However, AUG is also the translation initiation codon. When the codon is used for initiation, fMet is used instead of methionine, thereby forming the first amino acid of the nascent peptide chain. When the same codon appears later in the mRNA, normal methionine is used. Many organisms use variations of this basic mechanism.
The addition of the formyl group to methionine is catalyzed by the enzyme methionyl-tRNA formyltransferase. This modification is done after methionine has been loaded onto tRNA by aminoacyl-tRNA synthetase.
Note that methionine can be loaded either onto tRNA or tRNA. However, transformylase will catalyze the addition of the formyl group to methionine only if methionine has been loaded onto tRNA and NOT onto tRNA.
This methionine is removed from majority of proteins (both host and recombinant) by methionine aminopeptidase (MAP).
Relevance to immunology
Because fMet is present in proteins made by prokaryotes but not in those made by eukaryotes, the immune system can use it to help distinguish self from non-self. Polymorphonuclear cells can bind proteins starting with N-Formylmethionine, and use them to initiate phagocytosis.
References
- Sherman F, Stewart JW, Tsunasawa S (1985). "Methionine or not methionine at the beginning of a protein". BioEssays : News and Reviews in Molecular, Cellular and Developmental Biology. 3 (1): 27–31. doi:10.1002/bies.950030108. PMID 3024631.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Immunology at MCG 1/phagstep
- "The Innate Immune System: Pattern-Recognition Receptors, Antigen-Nonspecific Antimicrobial Body Molecules, and Cytokines".
- Detmers PA, Wright SD, Olsen E, Kimball B, Cohn ZA (1987). "Aggregation of complement receptors on human neutrophils in the absence of ligand". The Journal of Cell Biology. 105 (3): 1137–45. PMC 2114803. PMID 2958480.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- N-Formylmethionine at the U.S. National Library of Medicine Medical Subject Headings (MeSH)