This is an old revision of this page, as edited by ChuispastonBot (talk | contribs) at 19:21, 13 October 2011 (r2.7.1) (Robot: Adding pt:Tetraborano). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 19:21, 13 October 2011 by ChuispastonBot (talk | contribs) (r2.7.1) (Robot: Adding pt:Tetraborano)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)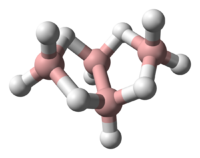
| |
| Names | |
|---|---|
| IUPAC names
tetraborane(10) arachno-B4H10 | |
| Identifiers | |
| CAS Number | |
| ChEBI | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
InChI
| |
| Properties | |
| Chemical formula | B4H10 |
| Molar mass | 53.32 g/mol |
| Appearance | colourless gas |
| Density | 2.3 kg m (gas) |
| Melting point | −120.8 °C |
| Boiling point | 18 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Tetraborane, or to be more precise tetraborane(10) or arachno-B4H10 was the first boron hydride compound to be classified by Stock and Messenez in 1912 and was first isolated by Alfred Hock. It has a relatively low boiling point at 18°C and is liquid at room temperature. Tetraborane gas is foul smelling and toxic.
Safety
Because it is easily oxidized it must be kept under vacuum. Tetraborane ignites when it comes in contact with air, oxygen, and nitric acid. Boranes in general including tetraborane have been deemed very toxic and are biologically destructive. A study consisting of small daily exposure of the chemical to rabbits and rats resulted in fatality
Preparation
Tetraborane can be produced via a reaction between acid and magnesium, aluminum, or beryillium borides. Hydrolysis of magnesium boride, hydrogenation of of boron halide (at high temperatures) and the pyrolysis of diborane also produce tetraborane. The hydrolysis of magnesium boride was one of the first reactions to give a high yield(14%) of tetraborane. Phosphoric acid proved to be the most efficient acid (other than hydrochloric and sulfuric acid)in the reaction with magnesium boride. Reduction of boron halides with hydrogen in the presence of metal hydride at high temperatures also produces tetraborane
References
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, Florida: CRC Press. p. B-84. ISBN 0-8493-0462-8..
- http://voh.chem.ucla.edu/vohtar/spring05/classes/172/pdf/p21-30Borane.pdf
- Dain, C. J., Downs, A. J., Laurenson, G. S., & Rankin, D. W. (1981). The Molecular Structure of Tetraborane(10) in the Gas Phase as determined by a Joint Analysis of Electron-diffraction and Microwave Data. Journal of the Chemical Society, 472-477.
http://pubs.acs.org/doi/pdf/10.1021/ic049558o3 http://voh.chem.ucla.edu/vohtar/spring05/classes/172/pdf/p21-30Borane.pdf
External links
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |