This is an old revision of this page, as edited by 96.38.238.10 (talk) at 18:38, 22 October 2011. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
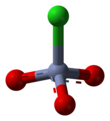
Revision as of 18:38, 22 October 2011 by 96.38.238.10 (talk)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| Chemical structure of the Pyridinium Chlorochromate | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Pyridinium chlorochromate | |||
| Other names PCC | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.043.253 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C5H5NHClCrO3 | ||
| Molar mass | 215.56 g/mol | ||
| Appearance | orange crystalline powder | ||
| Melting point | 205 °C (401 °F; 478 K) | ||
| Solubility in other solvents | soluble in dichloromethane, benzene, diethyl ether, acetone, acetonitrile, THF | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | oxidizing, toxic, flammable carcinogenic, irritant | ||
| NFPA 704 (fire diamond) |
 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Pyridinium chlorochromate is a reddish orange solid reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Pyridinium chlorochromate, or PCC, will not fully oxidize a primary alcohol to the carboxylic acid as does the Jones reagent. A disadvantage to using PCC is its toxicity. PCC was developed by Elias James Corey and William Suggs in 1975.
Pyridinium dichromate is a similar oxidizing agent, which has the advantage of being less acidic.
Preparation
The original preparation by Corey involves adding one equivalent of pyridine to a solution of one equivalent of chromic acid and concentrated hydrochloric acid:
- C5H5N + HCl + CrO3 →
Agarwal et al. presented an alternative synthesis that avoids the harmful side product chromyl chloride (CrO2Cl2). Chromium(VI) oxide is treated with pyridinium chloride:
- Cl + CrO3 →
Properties and uses
PCC is primarily used as an oxidant. In particular, it has proven to be highly effective in oxidizing primary and secondary alcohols to aldehydes and ketones, respectively. Rarely does over-oxidation occur (whether intentionally or accidentally) to form carboxylic acids. A typical PCC oxidation involves addition of the alcohol to a suspension of PCC in dichloromethane. A sample reaction would be:
- C5H5NHCrO3Cl + R2CHOH → C5H5NHCl + H2CrO3 + R2C=O
In practice the chromium byproduct deposits with pyridine as a sticky black tar, which can complicate matters. Addition of an inert adsorbent such as crushed molecular sieves or silica gel allows the sticky byproduct to adsorb to the surface, and makes workup easier.
In addition to simple oxidations of hydroxyl groups, rearrangements are possible. For example, tertiary alcohols cannot be oxidized directly. However, in the Babler oxidation, the chromate ester formed with PCC and an allylic tertiary alcohol can isomerize via a -sigmatropic reaction before the carbonyl-forming oxidation step. Other common oxidants usually lead to simple dehydration rather than any oxidation reaction at tertiary hydroxyl centers.
Another notable oxidative reaction of PCC is its efficient conversion of unsaturated alcohols or aldehydes to cyclohexenones. This particular pathway is known as oxidative cationic cyclization.
Controversy
PCC is controversial as it contains chromium(VI), a known carcinogen. Other methods for oxidizing alcohols using less toxic reagents have been introduced and are favored by green chemists:
- DMSO-based oxidations (Swern oxidation, Moffatt oxidation)
- hypervalent iodine based oxidation (such as the Dess-Martin periodinane)
References
- ^ Corey, E.J.; Suggs, W. (1975). "Pyridinium Chlorochromate. An Efficient Reagent for Oxidation of Primary and Secondary Alcohols to Carbonyl Compounds". Tetrahedron Lett. 16 (31): 2647–2650. doi:10.1016/S0040-4039(00)75204-X.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Agarwal, S; Tiwari, H. P.; Sharma, J. P. (1990). "Pyridinium Chlorochromate: an Improved Method for its Synthesis and use of Anhydrous acetic acid as catalyst for oxidation reactions". Tetrahedron. 46 (12): 4417–4420. doi:10.1016/S0040-4020(01)86776-4.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Paquette, L. A.; Earle, M. J.; Smith, G. F. (1998). "(4R)-(+)-tert-Butyldimethylsiloxy-2-cyclopenten-1-one". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 9, p. 132. - Tu, Y.; Frohn, M.; Wang, Z.-X.; Shi, Y. (2003). "Synthesis of 1,2:4,5-Di-O-Isopropylidene-D-erythro-2,3-hexodiulo-2,6-pyranose. A highly Enantioselective Ketone Catalyst for Epoxidation". Organic Syntheses. 80: 1
{{cite journal}}: CS1 maint: multiple names: authors list (link). - White, J. D.; Grether, U. M.; Lee, C.-S. (2005). "(R)-(+)-3,4-Dimethylcyclohex-2-en-1-one". Organic Syntheses. 82: 108
{{cite journal}}: CS1 maint: multiple names: authors list (link).
Further reading
- G. Tojo and M. Fernâandez (2006). Oxidation of alcohols to aldehydes and ketones : a guide to current common practice. New York: Springer. ISBN 0387236074.
External links
- IARC Monograph "Chromium and Chromium compounds"
- History of PCC
- Datasheet PDC
- National Pollutant Inventory - Chromium VI and compounds fact sheet