This is an old revision of this page, as edited by Beetstra (talk | contribs) at 09:13, 24 October 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 09:13, 24 October 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Lithium aluminium hydride | |||
| Systematic IUPAC name Lithium alumanuide | |||
| Other names
Lithal Lithium alanate | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) | |||
| Abbreviations | LAH | ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.037.146 | ||
| EC Number |
| ||
| Gmelin Reference | 13167 | ||
| PubChem CID | |||
| RTECS number |
| ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | LiAlH4 | ||
| Molar mass | 37.95 g/mol | ||
| Appearance | white crystals (pure samples) grey powder (commercial material) hygroscopic | ||
| Density | 0.917 g/cm, solid | ||
| Melting point | 150 °C (423 K), decomposing | ||
| Solubility in water | reactive | ||
| Structure | |||
| Crystal structure | monoclinic | ||
| Space group | P21c | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | highly flammable | ||
| GHS labelling: | |||
| Pictograms | 
| ||
| Signal word | Danger | ||
| Hazard statements | H260 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 125 °C | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for the hydrogen storage.
Properties, structure, preparation

LAH is a white solid, but commercial samples are usually gray due to contamination. This material can be purified by recrystallization from diethyl ether. Large-scale purifications employ a Soxhlet extractor. Commonly, the impure gray material is used in synthesis, since the impurities are innocuous and can be easily separated from the organic products. The pure powdered material is pyrophoric, but not its large crystals. Some commercial materials contain mineral oil to inhibit reactions with atmospheric moisture, but more commonly it is packed in moisture-proof plastic sacks.
LAH violently reacts with water, including atmospheric moisture. The reaction proceeds according the following idealized equation:
- LiAlH4 + 4 H2O → LiOH + Al(OH)3 + 4 H2
This reaction provides a useful method to generate hydrogen in the laboratory. Aged, air-exposed samples often appear white because they have absorbed enough moisture to generate a mixture of the white compounds lithium hydroxide and aluminium hydroxide.
Structure
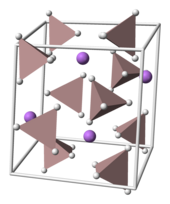
LAH crystallizes in the monoclinic space group P21c. The unit cell is defined as follows: a = 4.82, b = 7.81, and c = 7.92 Å, α = γ = 90° and β = 112°. The solid consists of Li centers surrounded by five AlH
4 tetrahedra. The Li centers are bonded to one hydrogen atom from each of the surrounding tetrahedra creating a bipyramid arrangement. At high pressures (>2.2 GPa) a phase transition may occur to give β-LAH.
Preparation
LAH was first prepared from the reaction between lithium hydride (LiH) and aluminium chloride:
- 4 LiH + AlCl3 → LiAlH4 + 3 LiCl
In addition to this method, the industrial synthesis entails the initial preparation of sodium aluminium hydride from the elements under high pressure and temperature:
- Na + Al + 2 H2 → NaAlH4
LAH is then prepared by metathesis reaction according to:
- NaAlH4 + LiCl → LiAlH4 + NaCl
which proceeds in a high yield of LAH. LiCl is removed by filtration from an ethereal solution of LAH, with subsequent precipitation of LAH to yield a product containing around 1% w/w LiCl.
Solubility data
| Temperature (°C) | |||||
| Solvent | 0 | 25 | 50 | 75 | 100 |
| Diethyl ether | – | 5.92 | – | – | – |
| THF | – | 2.96 | – | – | – |
| Monoglyme | 1.29 | 1.80 | 2.57 | 3.09 | 3.34 |
| Diglyme | 0.26 | 1.29 | 1.54 | 2.06 | 2.06 |
| Triglyme | 0.56 | 0.77 | 1.29 | 1.80 | 2.06 |
| Tetraglyme | 0.77 | 1.54 | 2.06 | 2.06 | 1.54 |
| Dioxane | – | 0.03 | – | – | – |
| Dibutyl ether | – | 0.56 | – | – | – |
LAH is soluble in many etheral solutions. However, it may spontaneously decompose due to the presence of catalytic impurities, though, it appears to be more stable in tetrahydrofuran (THF). Thus, THF is preferred over, e.g., diethyl ether, despite the lower solubility.
Thermodynamic data
The table summarizes thermodynamic data for LAH and reactions involving LAH, in the form of standard enthalpy, entropy and Gibbs free energy change, respectively.
| Reaction | ΔH° (kJ/mol) |
ΔS° (J/(mol·K)) |
ΔG° (kJ/mol) |
Comment |
|---|---|---|---|---|
| Li (s) + Al (s) + 2 H2(g) → LiAlH4 (s) | −116.3 | −240.1 | −44.7 | Standard formation from the elements. |
| LiH (s) + Al (s) + 3/2 H2 (g) → LiAlH4 (s) | −25.6 | −170.2 | 23.6 | Using ΔH°f(LiH) = −90.5, ΔS°f(LiH) = −69.9, and ΔG°f(LiH) = −68.3. |
| LiAlH4 (s) → LiAlH4 (l) | 22 | – | – | Heat of fusion. Value might be unreliable. |
| LiAlH4 (l) → ⅓ Li3AlH6 (s) + ⅔ Al (s) + H2 (g) | 3.46 | 104.5 | −27.68 | ΔS° calculated from reported values of ΔH° and ΔG°. |
Thermal decomposition
LAH is metastable at room temperature. During prolonged storage it slowly decomposes to Li3AlH6 and LiH. This process can be accelerated by the presence of catalytic elements, such as titanium, iron or vanadium.

When heated LAH decomposes in a three-step reaction mechanism:
- 3 LiAlH4 → Li3AlH6 + 2 Al + 3 H2 (R1)
- 2 Li3AlH6 → 6 LiH + 2 Al + 3 H2 (R2)
- 2 LiH + 2 Al → 2 LiAl + H2 (R3)
R1 is usually initiated by the melting of LAH in the temperature range 150–170 °C, immediately followed by decomposition into solid Li3AlH6, although R1 is known to proceed below the melting point of LiAlH4 as well. At about 200 °C, Li3AlH6 decomposes into LiH (R2) and Al which subsequently convert into LiAl above 400 °C (R3). Reaction R1 is effectively irreversible. R3 is reversible with an equilibrium pressure of about 0.25 bar at 500 °C. R1 and R2 can occur at room temperature with suitable catalysts.
Applications
Use in organic chemistry
Lithium aluminium hydride is widely used in organic chemistry as a reducing agent. It is more powerful than the related reagent sodium borohydride due to the weaker Al-H bond compared to the B-H bond. Often as a solution in diethyl ether and followed by an acid work-up, it will convert esters, carboxylic acids, aldehydes, and ketones into the corresponding alcohols (see: carbonyl reduction). Similarly, it converts amide, nitro, nitrile, imine, oxime, and azide compounds into the amines (see: amide reduction). It reduces quaternary ammonium cations into the corresponding tertiary amines. Reactivity can be tuned by replacing hydride groups by alkoxy groups. Despite handling problems associated with its reactivity, it is even used at the small-industrial scale, although for large-scale reductions, the related reagent sodium bis(2-methoxyethoxy)aluminium hydride is more commonly used.
LAH is most commonly used for the reduction of esters and carboxylic acids to primary alcohols; prior to the advent of LiAlH4, this was a difficult conversion involving sodium metal in boiling ethanol (the Bouveault-Blanc reduction). Aldehydes and ketones can also be reduced to alcohols by LAH, but this is usually done using milder reagents such as NaBH4; α,β-unsaturated ketones are reduced to allylic alcohols. When epoxides are reduced using LAH, the reagent attacks the less hindered end of the epoxide, usually producing a secondary or tertiary alcohol. Epoxycyclohexanes are reduced to give axial alcohols preferentially.
Partial reduction of acid chlorides to give the corresponding aldehyde product cannot proceed via LAH, since the latter reduces all the way to the primary alcohol. Instead, the milder lithium aluminium tri(t-butoxy)hydride must be used, which reacts significantly faster with the acid chloride than with the aldehyde. For example, when isovaleric acid is treated with thionyl chloride to give isovaleroyl chloride, it can then be reduced via lithium aluminium tri(t-butoxy)hydride to give isovaleraldehyde in 65% yield.

Using LAH, amines can be prepared by the reduction of amides, oximes, nitriles, nitro compounds or alkyl azides.
Lithium aluminium hydride also reduces alkyl halides to alkanes, although this reaction is rarely employed. Alkyl iodides react the fastest, followed by alkyl bromides and then alkyl chlorides. Primary halides are the most reactive followed by secondary halides. Tertiary halides react only in certain cases.
Lithium aluminium hydride does not reduce simple alkenes, arenes, and alkynes are only reduced if an alcohol group is nearby.
Inorganic chemistry
LAH is widely used to prepare main group and transition metal hydrides from the corresponding metal halides. For example, sodium hydride (NaH) can be prepared from sodium chloride (NaCl) through the following reaction:
- LiAlH4 + 4 NaCl → 4 NaH + LiCl + AlCl3
LAH also reacts with many inorganic ligands to form coordinated alumina complexes associated with lithium ions.
- LiAlH4 + NH3 → Li
Hydrogen storage

LiAlH4 contains 10.6 wt% hydrogen thereby making LAH a potential hydrogen storage medium for future fuel cell powered vehicles. The high hydrogen content, as well as the discovery of reversible hydrogen storage in Ti-doped NaAlH4, have sparked renewed research into LiAlH4 during the last decade. A substantial research effort has been devoted to accelerating the decomposition kinetics by catalytic doping and by ball milling. In order to take advantage of the total hydrogen capacity, the intermediate compound LiH must be dehydrogenated as well. Due to its high thermodynamic stability this requires temperatures in excess of 400 °C which is not considered feasible for transportation purposes. Accepting LiH + Al as the final product, the hydrogen storage capacity is reduced to 7.96 wt%. Another problem related to hydrogen storage is the recycling back to LiAlH4 which, due to its relatively low stability, requires an extremely high hydrogen pressure in excess of 10000 bar. Cycling only reaction R2, that is using Li3AlH6 as starting material, would store 5.6 wt% hydrogen in a single step (vs. two steps for NaAlH4 which stores about the same amount of hydrogen). However, attempts on this have not been successful so far.
Other tetrahydridoaluminiumates
A variety of salts analogous to LAH are known. NaH can be used to efficiently produce sodium aluminium hydride (NaAlH4) by metathesis in THF:
- LiAlH4 + NaH → NaAlH4 + LiH
Potassium aluminium hydride (KAlH4) can be produced similarly in diglyme as a solvent:
- LiAlH4 + KH → KAlH4 + LiH
The reverse, i.e., production of LAH from either sodium aluminium hydride or potassium aluminium hydride can be achieved by reaction with LiCl or lithium hydride in diethyl ether or THF:
- NaAlH4 + LiCl → LiAlH4 + NaCl
- KAlH4 + LiCl → LiAlH4 + KCl
"Magnesium alanate" (Mg(AlH4)2) arises similarly using MgBr2:
- 2 LiAlH4 + MgBr2 → Mg(AlH4)2 + 2 LiBr
Red-Al (or SMEAH, NaAlH2(OC2H4OCH3)2) is synthesized by reacting sodium aluminum tetrahydride (NaAlH4) and 2-methoxyethanol:
See also
References
- Index no. 001-002-00-4 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Official Journal of the European Union L353, 31 December 2008, pp. 1–1355 at p 340.
- ^ Finholt, A. E.; Bond, A. C.; Schlesinger, H. I. (1947). Journal of the American Chemical Society. 69 (5): 1199. doi:10.1021/ja01197a061.
{{cite journal}}: Missing or empty|title=(help) - ^ Gerrans, G.C. and Hartmann-Petersen, P. (2007). Sasol Encyclopaedia of Science and Technology. New Africa Books. p. 143. ISBN 1869283848.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Keese, Reinhart; Brändle, Martin and Toube, Trevor Philip (2006). Practical organic synthesis: a student's guide. John Wiley and Sons. p. 134. ISBN 0470029668.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Andreasen, A.; Vegge, T.; Pedersen, A.S. (2005). "Dehydrogenation kinetics of as-received and ball-milled LiAlH4" (PDF). Journal of Solid State Chemistry. 178 (12): 3672. doi:10.1016/j.jssc.2005.09.027.
- Pohanish, Richard P. (2008). Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens (5th ed.). William Andrew Publishing. p. 1540. ISBN 978-0-8155-1553-1.
- Løvvik, O.M.; Opalka, S.M.; Brinks, H.W.; Hauback, B.C. (2004). "Crystal structure and thermodynamic stability of the lithium alanates LiAlH4 and Li3AlH6". Physical Review B. 69 (13): 134117. doi:10.1103/PhysRevB.69.134117.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Holleman, A. F., Wiberg, E., Wiberg, N. (2007). Lehrbuch der Anorganischen Chemie, 102nd ed. de Gruyter. ISBN 978-3-11-017770-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Mikheeva, V. I.; Troyanovskaya, E. A. (1971). "Solubility of lithium aluminum hydride and lithium borohydride in diethyl ether". Bulletin of the Academy of Sciences of the USSR Division of Chemical Science. 20 (12): 2497. doi:10.1007/BF00853610.
- ^ Patnaik, Pradyot (2003). Handbook of Inorganic Chemicals. McGraw-Hill. p. 492. ISBN 978-0-07-049439-8.
- Smith, Martin B.; Bass, George E. (1963). "Heats and Free Energies of Formation of the Alkali Aluminum Hydrides and of Cesium Hydride". Journal of Chemical & Engineering Data. 8 (3): 342. doi:10.1021/je60018a020.
- ^ Dymova T. N.; Aleksandrov, D. P.; Konoplev, V. N.; Silina,T. A.; Sizareva; A. S. (1994). Russ. J. Coord. Chem. 20: 279.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - Dilts, J. A.; Ashby, E. C. (1972). "Thermal decomposition of complex metal hydrides". Inorganic Chemistry. 11 (6): 1230. doi:10.1021/ic50112a015.
- ^ Blanchard, D; Brinks, H; Hauback, B; Norby, P (2004). "Desorption of LiAlH4 with Ti- and V-based additives". Materials Science and Engineering B. 108: 54. doi:10.1016/j.mseb.2003.10.114.
- Chen, Jun; Kuriyama, Nobuhiro; Xu, Qiang; Takeshita, Hiroyuki T.; Sakai, Tetsuo (2001). "Reversible Hydrogen Storage via Titanium-Catalyzed LiAlH4and Li3AlH6". The Journal of Physical Chemistry B. 105 (45): 11214. doi:10.1021/jp012127w..
- Balema, V; Pecharsky, V.K; Dennis, K.W (2000). "Solid state phase transformations in LiAlH4 during high-energy ball-milling". Journal of Alloys and Compounds. 313: 69. doi:10.1016/S0925-8388(00)01201-9.
- ^ Andreasen, A (2006). "Effect of Ti-doping on the dehydrogenation kinetic parameters of lithium aluminum hydride". Journal of Alloys and Compounds. 419: 40. doi:10.1016/j.jallcom.2005.09.067.
- Andreasen, A; Pedersen, A S; Vegge, T (2005). "Dehydrogenation kinetics of as-received and ball-milled LiAlH4". Journal of Solid State Chemistry. 178 (12): 3672. doi:10.1016/j.jssc.2005.09.027.
- Balema, V; Wiench, J. W.; Dennis, K. W.; Pruski, M.; Pecharsky, V. K. (2001). "Titanium catalyzed solid-state transformations in LiAlH4 during high-energy ball-milling". Journal of Alloys and Compounds. 329: 108. doi:10.1016/S0925-8388(01)01570-5.
- Brown, H. C. (1951). Org. React. 6: 469.
{{cite journal}}: Missing or empty|title=(help) - "Red-Al, Sodium bis(2-methoxyethoxy)aluminumhydride".
- Reetz, M. T.; Drewes, M. W.; Schwickardi, R. Organic Syntheses, Coll. Vol. 10, p.256 (2004); Vol. 76, p.110 (1999). (Article)
- Oi, R.; Sharpless, K. B. Organic Syntheses, Coll. Vol. 9, p.251 (1998); Vol. 73, p.1 (1996). (Article)
- Koppenhoefer, B.; Schurig, V. Organic Syntheses, Coll. Vol. 8, p.434 (1993); Vol. 66, p.160 (1988). (Article)
- Barnier, J. P.; Champion, J.; Conia, J. M. Organic Syntheses, Coll. Vol. 7, p.129 (1990); Vol. 60, p.25 (1981). (Article)
- Elphimoff-Felkin, I.; Sarda, P. Organic Syntheses, Coll. Vol. 6, p.769 (1988); Vol. 56, p.101 (1977). (Article)
- Rickborn, Bruce; Quartucci, Joe (1964). "Stereochemistry and Mechanism of Lithium Aluminum Hydride and Mixed Hydride Reduction of 4-t-Butylcyclohexene Oxide". The Journal of Organic Chemistry. 29 (11): 3185. doi:10.1021/jo01034a015.
- Wade, L. G. Jr., Organic Chemistry, 6th edition (Pearson Prentice Hall, 2006, ISBN 0-13-147871-0)
- Seebach, D.; Kalinowski, H.-O.; Langer, W.; Crass, G.; Wilka, E.-M. Organic Syntheses, Coll. Vol. 7, p.41 (1990). (Article)
- Park, C. H.; Simmons, H. E. Organic Syntheses, Coll. Vol. 6, p.382 (1988); Vol. 54, p.88 (1974). (Article)
- Chen, Y. K.; Jeon, S.-J.; Walsh, P. J.; Nugent, W. A. Organic Syntheses, Vol. 82, p.87 (2005). (Article)
- Johnson, J. Enoch; Blizzard, Ronald H.; Carhart, Homer W. (1948). "Hydrogenolysis of alkyl halides by lithium aluminum hydride". Journal of the American Chemical Society. 70 (11): 3664. doi:10.1021/ja01191a035. PMID 18121883.
- Krishnamurthy, S.; Brown, Herbert C. (1982). "Selective reductions. 28. The fast reaction of lithium aluminum hydride with alkyl halides in THF. A reappraisal of the scope of the reaction". The Journal of Organic Chemistry. 47 (2): 276. doi:10.1021/jo00341a018.
- Carruthers, W. (2004). Some modern methods of organic synthesis. Cambridge University Press. p. 470. ISBN 0521311179.
- Wender, P. A.; Holt, D. A.; Sieburth, S. Mc N. Organic Syntheses, Coll. Vol. 7, p.456 (1990); Vol. 64, p.10 (1986). (Article)
- Bogdanovic, B; Schwickardi, M (1997). "Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials". Journal of Alloys and Compounds. 253–254: 1. doi:10.1016/S0925-8388(96)03049-6.
- ^ Varin, R A; Czujko, T; Wronski, Z S (2009). Nanomaterials for Solid State Hydrogen Storage (5th ed.). Springer. p. 338. ISBN 978-0-387-77711-5.
- ^ Santhanam, Ranganathan; Sean Mcgrady, G. (2008). "Synthesis of alkali metal hexahydroaluminate complexes using dimethyl ether as a reaction medium". Inorganica Chimica Acta. 361 (2): 473. doi:10.1016/j.ica.2007.04.044.
- Wiberg, Egon; Wiberg, Nils and Holleman, Arnold Frederick (2001). Inorganic chemistry. Academic Press. p. 1056. ISBN 0123526515.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Casensky, B.; Machacek, J.; Abrham, K. (1971). Collect. Czech. Chem. Commun. 36: 2648.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link)
Further reading
- Wiberg, Egon & Amberger, Eberhard (1971). Hydrides of the elements of main groups I-IV. Elsevier. ISBN 0-444-40807-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Hajos, Andor (1979). Complex Hydrides and Related Reducing Agents in Organic Synthesis. Elsevier. ISBN 0-444-99791-1.
- Lide (ed.), David R. (1997). Handbook of chemistry and physics. CRC Press. ISBN 0-8493-0478-4.
- Carey, Francis A. (2002). Organic Chemistry with Online Learning Center and Learning by Model CD-ROM. McGraw-Hill. ISBN 0-07-252170-8. on-line version
- Chapter 5 in Andreasen, Anders (2005). Hydrogen Storage Materials with Focus on Main Group I-II Elements. Risoe National Laboratory. ISBN 87-550-3498-5. Full text version
External links
- Usage of LiAlH4 in Organic Syntheses
- Condensed phase thermochemistry data from Nist webbook
- Materials Safety Data Sheet from Cornell University
- Sandia National Laboratory - Hydride information center
- Synthesis of LAH
- Reduction reactions, University of Birmingham, Teaching Resources - 4th Year
- PubChem LiAlH4 summary
Template:Link GA Template:Link FA
Categories: