This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 10:51, 24 October 2011 (Updating {{chembox}} (changes to verified and watched fields - updated 'DrugBank_Ref', 'UNII_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or bugs)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 10:51, 24 October 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (changes to verified and watched fields - updated 'DrugBank_Ref', 'UNII_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or bugs))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)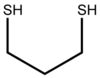
| |
| Names | |
|---|---|
| IUPAC name Propane-1,3-dithiol | |
| Other names 1,3-dimercaptopropane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.371 |
| RTECS number |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H8S2 |
| Molar mass | 108.22 g·mol |
| Appearance | Colorless liquid |
| Density | 1.078 g/cm³ |
| Melting point | −79 °C (−110 °F; 194 K) |
| Boiling point | 169 °C (336 °F; 442 K) |
| Solubility in water | slight |
| Solubility in solvents | all organic solvents |
| Refractive index (nD) | 1.539 |
| Structure | |
| Dipole moment | 0 D |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | stench |
| Flash point | 138 °F |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
1,3-Propanedithiol is the chemical compound with the formula HSCH2CH2CH2SH. This dithiol is a useful reagent in organic synthesis. This liquid, which is readily available commercially, has an intense stench.
Use in organic synthesis
1,3-Propanedithiol is mainly used for the protection of aldehydes and ketones via their reversible formation of dithianes. A prototypical reaction is its formation of 1,3-dithiane from formaldehyde. The reactivity of this dithiane illustrates the concept of umpolung.
The unpleasant odour of 1,3-propanedithiol has encouraged the development of alternative reagents that generate similar derivatives.
Use in inorganic synthesis
1,3-Propanedithiol reacts with metal ions to form chelate rings. Illustrative is the synthesis of the diiron derivative:
Safety
The stench of 1,3-propanedithiol can be neutralized with bleach.
See also
References
- Conrow, R. E.; Le Huérou, Y. (2004). "1,3-Propanedithiol". Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette). J. Wiley & Sons, New York. doi:10.1002/047084289.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - Corey, E. J.; Seebach, D. (1988). "1,3-Dithiane". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 6, p. 556. - Liu, Q.; Che, G. Yu, H.; Liu, Y.; Zhang, J. Zhang, Q.; Dong, D. (2003). "The First Nonthiolic, Odorless 1,3-Propanedithiol Equivalent and Its Application in Thioacetalization". Journal of Organic Chemistry. 68 (23): 9148–9150. doi:10.1021/jo034702t. PMID 14604400.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Winter, A.; Zsolnai, L. and Huttner, G. (1982). "Zweikernige und dreikernige Carbonyleisenkomplexe mit 1,2- und 1,3-Dithiolatobrückenliganden". Zeitschrift für Naturforschung. 37b: 1430–1436.
{{cite journal}}: CS1 maint: multiple names: authors list (link)