This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 13:18, 25 October 2011 (Updating {{chembox}} (changes to verified fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or [[user...). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:18, 25 October 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (changes to verified fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or [[user...)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)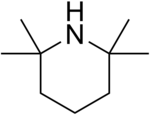
| |
| Names | |
|---|---|
| IUPAC name 2,2,6,6-Tetramethylpiperidine | |
| Other names
Norpempidine Tetramethylpiperidine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | TMP |
| ChemSpider | |
| ECHA InfoCard | 100.011.090 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H19N |
| Molar mass | 141.254 g/mol |
| Appearance | Clear liquid |
| Density | 0.83 g/mL |
| Melting point | −59 °C (−74 °F; 214 K) |
| Boiling point | 152 °C (306 °F; 425 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
2,2,6,6-Tetramethylpiperidine or TMP or HTMP is a clear liquid with an amine odor. This amine is used in chemistry as a hindered base (hindered amine) because it can dissolve in organic solvents unlike ordinary bases such as potassium hydroxide.
There are many ways to synthesise TMP. One recent method starts with a conjugate addition reaction of ammonia to phorone. The intermediate triacetone amine is then reduced in a Wolff-Kishner reaction.

TMP is the starting material for an even stronger base lithium tetramethylpiperidide and the radical species TEMPO. Another non-nucleophilic base is N,N-diisopropylethylamine.
See also
References
- Detlef Kampmann, Georg Stuhlmüller, Roger Simon, Fabrice Cottet, Frédéric Leroux, Manfred Schlosser (2005). "A Large-Scale Low-Cost Access to the Lithium 2,2,6,6-Tetramethylpiperidide Precursor". Synthesis. 2005 (06): 1028–1029. doi:10.1055/s-2004-834856.
{{cite journal}}: CS1 maint: multiple names: authors list (link)