This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 12:01, 1 November 2011 (Updating {{drugbox}} (changes to verified fields - added verified revid - updated '') per Chem/Drugbox validation (report errors or bugs)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:01, 1 November 2011 by CheMoBot (talk | contribs) (Updating {{drugbox}} (changes to verified fields - added verified revid - updated '') per Chem/Drugbox validation (report errors or bugs))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound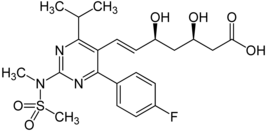 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Crestor |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603033 |
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 20% |
| Metabolism | Liver |
| Elimination half-life | 19 h |
| Excretion | Urine / Faeces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.216.011 |
| Chemical and physical data | |
| Formula | C22H28FN3O6S |
| Molar mass | 481.539 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Rosuvastatin (marketed by AstraZeneca as Crestor & marketed by Abbott Healthcare Pvt. Ltd. in India as 'R2') is a member of the drug class of statins, used to treat high cholesterol and related conditions, and to prevent cardiovascular disease. It was developed by Shionogi.
Medical uses
The primary uses of rosuvastatin is for the treatment of dyslipidemia. It is recommended to be used only after other measures such as diet, exercise, and weight reduction have not improved cholesterol levels.
Structure
Rosuvastatin has structural similarities with most other synthetic statins, e.g., atorvastatin, cerivastatin, pitavastatin, but rosuvastatin unusually also contains sulfur.
Crestor is actually rosuvastatin calcium, in which calcium replaces the H in the carboxylic acid group on the right of the 2 structure diagrams.
Mechanism of action
Further information: ]Rosuvastatin is a competitive inhibitor of the enzyme HMG-CoA reductase, having a mechanism of action similar to that of other statins. Its approximate elimination half life is 19 hours and its time to peak plasma concentration is reached in 3–5 hours following oral administration.
Putative beneficial effects of rosuvastatin therapy on chronic heart failure may be negated by increases in collagen turnover markers as well as a reduction in plasma coenzyme Q10 (CoQ10) levels in patients with chronic heart failure.
Indications and regulation
Rosuvastatin is approved for the treatment of high LDL cholesterol (dyslipidemia), total cholesterol (hypercholesterolemia), and/or triglycerides (hypertriglyceridemia). In February 2010, rosuvastatin was approved by the FDA for the primary prevention of cardiovascular events.
As of 2004, rosuvastatin had been approved in 154 countries and launched in 56. Approval in the United States by the FDA came on August 12, 2003.
The results of the JUPITER trial (2008) suggested that rosuvastatin may decrease the relative risk of heart attack and stroke in patients without hyperlipidemia but with elevated levels of highly-sensitive C-reactive protein. This could strongly impact medical practice by placing many patients on statin prophylaxis that otherwise would have been untreated. As a result of this clinical trial, the FDA approved rosuvastatin for the primary prevention of cardiovascular events.
The AURORA trial randomized 2776 patients undergoing hemodialysis due to kidney damage to receive either rosuvastatin or placebo. The randomized, double-blind study (2005 to 2009) found no difference in the two groups in the primary end-point, a combination of cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke. The study found no difference in all-cause mortality among this population at a mean follow-up of 3.8 years.
Effects on cholesterol levels
The effects of rosuvastatin on LDL cholesterol are dose-related. At the 10 mg dose, the average LDL cholesterol reduction was found to be 46% in one trial. Increasing the dose from 10 mg to 40 mg gave a modest additional 9% absolute reduction in LDL levels (55% below baseline levels).
FDA advisory for Asian patients
According to the FDA, the risk of myopathy during rosuvastatin therapy may be increased in Asian-Americans:
Because Asians appear to process the drug differently, half the standard dose can have the same cholesterol-lowering benefit in those patients, though a full dose could increase the risk of side-effects, a study by the drug's manufacturer, AstraZeneca, indicated.
Therefore, physicians should start Asian patients at the lowest dose level.
Marketing and competition
Patent protection
The main patent protecting rosuvastatin (RE37,314 - due to expire in 2016) was challenged as being an improper reissue of an earlier patent. This challenge was rejected in 2010, confirming protection until 2016.
Marketing
The drug was billed as a super-statin during its clinical development; the claim was that it offers high potency and improved cholesterol reduction compared to rivals in the class. The main competitors to rosuvastatin are atorvastatin (Lipitor) and simvastatin (Zocor). However, people can also combine ezetimibe with either rosuvastatin or atorvastatin and other agents on their own, for somewhat similar augmented response rates. So far, some published information for comparing rosuvastatin, atorvastatin, and ezetimibe/simvastatin results is available, but many of the relevant studies are still in progress.
First launched in 2003, sales of rosuvastatin were $129 million and $908 million in 2003 and 2004, respectively, with a total patient treatment population of over 4 million by the end of 2004. Typical per patient costs to the UK NHS are £18.03-26.02/month (compared to £0.85-1.37/month for simvastatin).
Debate & criticisms
In October 2003, several months after its introduction in Europe, Richard Horton, the editor of the medical journal The Lancet, criticized the way Crestor had been introduced. "AstraZeneca's tactics in marketing its cholesterol-lowering drug, rosuvastatin, raise disturbing questions about how drugs enter clinical practice and what measures exist to protect patients from inadequately investigated medicines," according to his editorial. The Lancet's editorial position is that the data for Crestor’s superiority relies too much on extrapolation from the lipid profile data (surrogate endpoints) and too little on hard clinical endpoints, which are available for other statins that had been on the market longer. The manufacturer responded by stating that few drugs had been tested so successfully on so many patients. In correspondence published in The Lancet, AstraZeneca's CEO Sir Tom McKillop called the editorial "flawed and incorrect" and slammed the journal for making "such an outrageous critique of a serious, well-studied medicine."
In 2004, the consumer interest organization Public Citizen filed a Citizen's Petition with the FDA, asking that Crestor be withdrawn from the US market. On March 11, 2005, the FDA issued a letter to Sidney M. Wolfe, M.D. of Public Citizen both denying the petition and providing an extensive detailed analysis of findings that demonstrated no basis for concerns about rosuvastatin compared with the other statins approved for marketing in the United States.
Myopathy
As with all statins, there is a concern of rhabdomyolysis, a severe undesired side-effect. The FDA has indicated that "it does not appear that the risk is greater with Crestor than with other marketed statins", but has mandated that a warning about this side-effect, as well as a kidney toxicity warning, be added to the product label.
Notes
- ^ "Crestor". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ^ "FDA Alert (03/2005) - Rosuvastatin Calcium (marketed as Crestor) Information". The Food and Drug Administration. March 14, 2005. Archived from the original on 2005-03-05. Retrieved 2005-03-20. - This page is subject to change; the date reflects the last revision date.
- ^ Nissen SE, Nicholls SJ, Sipahi I; et al. (2006). "Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial" (PDF). JAMA. 295 (13): 1556–65. doi:10.1001/jama.295.13.jpc60002. PMID 16533939.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Retrieved from package insert on 2009-03-11
- Ashton E, Windebank E, Skiba M; et al. (2010). "Why did high-dose rosuvastatin not improve cardiac remodeling in chronic heart failure? Mechanistic insights from the UNIVERSE study". Int J Cardiol. 146 (3): 404. doi:10.1016/j.ijcard.2009.12.028. PMID 20085851.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Core Data Sheet, Crestor Tablets" (PDF). AstraZeneca PLC. 2003. Retrieved 2005-03-20.
{{cite web}}: Unknown parameter|month=ignored (help) - NOTE: this is provider-oriented information and should not be used without the supervision of a physician. - ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2010/021366s016ltr.pdf
- "FDA Approves New Drug for Lowering Cholesterol". The Food and Drug Administration. August 12, 2003. Archived from the original on 2005-02-07. Retrieved 2005-03-20.
- Ridker PM, Danielson E; et al. (2008). "Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein". N. Engl. J. Med. 359 (21): 2195–207. doi:10.1056/NEJMoa0807646. PMID 18997196.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - Brendan M. Everett, MD, MPH; Robert J. Glynn, ScD; Jean G. MacFadyen, BA; Paul M Ridker, MD, MPH (2010). "Rosuvastatin in the Prevention of Stroke Among Men and Women With Elevated Levels of C-Reactive Protein" (PDF). Circulation. 121 (1): 143–150. doi:10.1161/CIRCULATIONAHA.109.874834. PMID 20026779.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Fellstrom BC, Jardine AG; et al. (2009). "Rosuvastatin and Cardiovascular Events in Patients Undergoing Hemodialysis". N. Engl. J. Med. 360 (14): 1395–1407. doi:10.1056/NEJMoa0810177. PMID 19332456.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, Cain VA, Blasetto JW. (2003). "Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR Trial)". Am J Cardiol. 92 (2): 152–60. doi:10.1016/S0002-9149(03)00530-7. PMID 12860216.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - FDA Advisory Targets Asian Patients (Los Angeles Times).
- Asian-Americans Among Groups More at Risk of Serious Muscle Damage (WebMD).
- "AstraZeneca's Crestor patent upheld;No generic competition until 2016".
- "CRESTOR Patent Upheld By US Court".
- Horton, Richard (2003). "The statin wars: why AstraZeneca must retreat". Lancet. 362 (9393): 1341. doi:10.1016/S0140-6736(03)14669-7. PMID 14585629.
{{cite journal}}: Unknown parameter|month=ignored (help)
McKillop T (2003). "The statin wars". Lancet. 362 (9394): 1498. doi:10.1016/S0140-6736(03)14698-3. PMID 14602449.{{cite journal}}: Unknown parameter|month=ignored (help) - Food and Drug Administration. "Docket No. 2004P-0113/CP1" (PDF).
References
- "Annual Report and Form 20-F, Information 2004" (PDF). AstraZeneca PLC. 2005.
- "Annual Report and Form 20-F, 2003" (PDF). AstraZeneca PLC. 2004. Archived from the original (PDF) on 2005-05-13. Retrieved 2005-03-20.
- "Highlights of Prescribing Information" (PDF). AstraZeneca PLC. 2008. Retrieved 2009-03-11.
- McTaggart F, Buckett L, Davidson R, Holdgate G, McCormick A, Schneck D, Smith G, Warwick M (2001). "Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor". Am J Cardiol. 87 (5A): 28B – 32B. doi:10.1016/S0002-9149(01)01454-0. PMID 11256847.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- "Rosuvastatin (Crestor) Information". eMedicineHealth. 2005-10-16.
- U.S. National Library of Medicine: Drug Information Portal - Rosuvastatin
| Lipid-lowering agents (C10) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI tract |
| ||||||||||||
| Liver |
| ||||||||||||
| Blood vessels |
| ||||||||||||
| Combinations | |||||||||||||
| Other | |||||||||||||
| |||||||||||||