This is an old revision of this page, as edited by Beetstra (talk | contribs) at 10:10, 3 November 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 10:10, 3 November 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound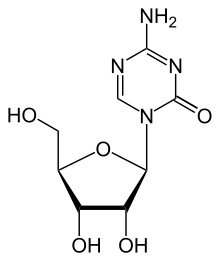 | |
| Clinical data | |
|---|---|
| Trade names | Vidaza |
| Other names | 5-azacytidine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607068 |
| Pregnancy category |
|
| Routes of administration | SubQ, IV |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | possible hepatic metabolism, mostly urinary excretion |
| Elimination half-life | 4 hr. |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.711 |
| Chemical and physical data | |
| Formula | C8H12N4O5 |
| Molar mass | 244.205 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Azacitidine (INN) or 5-azacytidine, sold under the trade name Vidaza, is a chemical analogue of cytidine, a nucleoside present in DNA and RNA. Azacitidine and its deoxy derivative, decitabine (also known as 5-aza-2′deoxycytidine), are used in the treatment of myelodysplastic syndrome. Both drugs were first synthesized in Czechoslovakia as potential chemotherapeutic agents for cancer.
Azacitidine has also been used as an experimental treatment in clinical trials involving cases of acute myeloid leukemia, where the patient has suffered more than one relapse- in these cases, standard chemotherapy, hematopoietic stem cell transplantation, and other mainline treatments have failed.
Uses
Azacitidine is mainly used in the treatment of myelodysplastic syndrome (MDS), for which it received approval by the U.S. Food and Drug Administration on May 19, 2004; it is marketed as Vidaza. In a randomized controlled trial comparing azacitidine to supportive treatment of MDS, around 16% of people receiving the drug had a complete or partial response—blood cell counts and bone marrow morphology returning to normal—and 2/3 patients who required blood transfusions before the study no longer needed them after receiving azacitidine.
It can also be used in vitro to remove methyl groups from DNA. This may weaken the effects of gene silencing mechanisms that occurred prior to the methylation. Methylation events are therefore believed to secure the DNA in a silenced state. Demethylation may reduce the stability of silencing signals and thus confer relative gene activation.
Mechanism of action
Methyltransferases in the presence of azacitidine incorporate it into DNA during replication and into RNA during transcription in the cell. Azacitidine acts as a false substrate and potent inhibitor of methyltransferases leading to reduction of DNA methylation — affecting the way cell regulation proteins are able to bind to the DNA/RNA substrate. Inhibition of DNA methylation occurs through the formation of stable complexes between the molecule and with DNA methyltransferases, thereby saturating cell methylation machinery.
See also
- DNA methylation, the phenomenon that azacitidine is known to interfere with
References
- WHO International Working Group for Drug Statistics Methodology (August 27, 2008). "ATC/DDD Classification (FINAL): New ATC 5th level codes". WHO Collaborating Centre for Drug Statistics Methodology. Archived from the original on 2008-05-06. Retrieved 2008-09-05.
- Deglin, Judith, & Vallerand, April. (2009). Davis's drug guide for nurses. Philadelphia: F.A. Davis Company. pg. 204-206
- Cihák A (1974). "Biological effects of 5-azacytidine in eukaryotes". Oncology. 30 (5): 405–22. doi:10.1159/000224981. PMID 4142650.
- Vidaza web site.
- Kaminskas E, Farrell AT, Wang Y-C, Sridhara R, Pazdur R (2005). "FDA Drug Approval Summary: Azacitidine (5-azacytidine, Vidaza) for Injectable Suspension". The Oncologist. 10 (3): 176–82. doi:10.1634/theoncologist.10-3-176. PMID 15793220.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Whitelaw E and Garrick D (2005), The Epigenome, Chapter 7, In: Mammalian Genomics, Ed: Ruvinsky A & Marshall Graves JA, CABI Publishing, Wallingford, UK, ISBN 0851999107.
External links
- Vidaza / Azacitidine Virtual Cancer Centre
- 5-Aza-2'-Deoxycytidine information and protocols to study DNA methylation
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |