This is an old revision of this page, as edited by Beetstra (talk | contribs) at 13:06, 7 November 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:06, 7 November 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff){{drugbox | verifiedrevid = 443868829 | IUPAC_name = 4--N-(4-methyl-3-{amino}phenyl)benzamide | image = Imatinib.svg
| tradename = Gleevec | Drugs.com = Monograph | MedlinePlus = a606018 | licence_EU = Glivec | licence_US = IMATINIB | pregnancy_AU = D | pregnancy_US = D | legal_AU = | legal_UK = POM | legal_US = Rx-only | routes_of_administration = Oral
| bioavailability = 98%
| protein_bound = 95%
| metabolism = Hepatic (mainly CYP3A4-mediated)
| elimination_half-life = 18 hours (imatinib)
40 hours (active metabolite)
| excretion = Fecal (68%) and renal (13%)
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 152459-95-5
| CAS_supplemental =
220127-57-1 (mesilate)
| ATC_prefix = L01
| ATC_suffix = XE01
| PubChem = 5291
| DrugBank_Ref =
| DrugBank = DB00619
| ChemSpiderID_Ref =
| ChemSpiderID = 5101
| UNII_Ref =
| UNII = BKJ8M8G5HI
| KEGG_Ref =
| KEGG = D08066
| ChEBI_Ref =
| ChEBI = 45783
| ChEMBL_Ref =
| ChEMBL = 941
| C=29 | H=31 | N=7 | O=1
| molecular_weight = 493.603 g/mol
589.7 g/mol (mesilate)
| smiles = Cc1ccc(cc1Nc2nccc(n2)c3cccnc3)NC(=O)c4ccc(cc4)CN5CCN(CC5)C
| InChI = 1/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34)
| InChIKey = KTUFNOKKBVMGRW-UHFFFAOYAJ
| StdInChI_Ref =
| StdInChI = 1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34)
| StdInChIKey_Ref =
| StdInChIKey = KTUFNOKKBVMGRW-UHFFFAOYSA-N
}}
Imatinib (originally STI571) is a drug used to treat certain types of cancer. It is currently marketed by Novartis as Gleevec (USA) or Glivec (Europe/Australia/Latin America) as its mesylate salt, imatinib mesilate (INN). It is used in treating chronic myelogenous leukemia (CML), gastrointestinal stromal tumors (GISTs) and some other diseases. By 2011, Gleevec has been FDA approved to treat ten different cancers. In CML, the tyrosine kinase enzyme ABL is locked in its activated form. It induces the abnormal phenotypes of CML: excessive proliferation and high white blood cell count. Imatinib binds to the site of tyrosine kinase activity, and prevents its activity, thereby causing tumor cell apoptosis.
Imatinib is the first member of a new class of agents that act by specifically inhibiting a certain enzyme that is characteristic of a particular cancer cell, rather than non-specifically inhibiting and killing all rapidly dividing cells, and served as a model for other targeted therapy modalities through tyrosine kinase inhibition.
History
Imatinib was developed in the late 1990s by biochemist Nicholas Lydon, a former researcher for Novartis, and oncologist Brian Druker of Oregon Health and Science University (OHSU). Other major contributions to imatinib development were made by Carlo Gambacorti-Passerini, a physician scientist and hematologist at University of Milano Bicocca, Italy, John Goldman at Hammersmith Hospital in London, UK, and later on by Charles Sawyers of Memorial Sloan-Kettering Cancer Center, who led the clinical trials confirming its efficacy in CML.
Imatinib was developed by rational drug design. After the Philadelphia chromosome mutation and hyperactive bcr-abl protein were discovered, the investigators screened chemical libraries to find a drug that would inhibit that protein. With high-throughput screening, they identified 2-phenylaminopyrimidine. This lead compound was then tested and modified by the introduction of methyl and benzamide groups to give it enhanced binding properties, resulting in imatinib.
Gleevec received FDA approval in May 2001. On the same month it made the cover of TIME magazine as the "magic bullet" to cure cancer. Druker, Lydon and Sawyers received the Lasker-DeBakey Clinical Medical Research Award in 2009 for "converting a fatal cancer into a manageable chronic condition".
Gleevec also holds the record for the drug with the fastest approval time by the FDA. According to Brian Druker, one of the developers of Imatinib, the biggest obstacle to being approved was the name of the drug. At that time, the drug was being called "Glivec", which is also the current spelling in most parts of the world. However, the United States Food and Drug Administration did not want people to mispronounce "Glivec" as "GLIE-VEC" which could be confused with a diabetic drug at the time. Therefore, Novartis, the pharmaceutical company who markets Gleevec, changed the name of "Glivec" to include two "e's" and avoid the phonetic confusion: Gleevec. Shortly thereafter, Gleevec was approved by the FDA.
Uses
Clinical
Chronic myelogenous leukemia
Imatinib is used in chronic myelogenous leukemia (CML), gastrointestinal stromal tumors (GISTs) and a number of other malignancies. One study demonstrated that imatinib mesylate was effective in patients with systemic mastocytosis, including those who had the D816V mutation in c-Kit. Experience has shown, however, that imatinib is much less effective in patients with this mutation, and patients with the mutation comprise nearly 90% of cases of mastocytosis. Early clinical trials also show its potential for treatment of hypereosinophilic syndrome and dermatofibrosarcoma protuberans.
In the United States, the Food and Drug Administration has approved imatinib as first-line treatment for CML. Imatinib has been shown to be more effective than the previous standard treatment of α-interferon and cytarabine.
Plexiform neurofibromas
For treatment of progressive plexiform neurofibromas associated with neurofibromatosis type I, early research has shown potential for using the c-kit tyrosine kinase blocking properties of imatinib.
Experimental
Imatinib may also have a role in the treatment of pulmonary hypertension. It has been shown to reduce both the smooth muscle hypertrophy and hyperplasia of the pulmonary vasculature in a variety of disease processes, including portopulmonary hypertension. In systemic sclerosis, the drug has been tested for potential use in slowing down pulmonary fibrosis. In laboratory settings, imatinib is being used as an experimental agent to suppress platelet-derived growth factor (PDGF) by inhibiting its receptor (PDGF-Rβ). One of its effects is delaying atherosclerosis in mice without or with diabetes.
Recent mouse animal studies at Emory University in Atlanta have suggested that imatinib and related drugs may be useful in treating smallpox, should an outbreak ever occur.
In vitro studies identified that a modified version of imatinib can bind to gamma-secretase activating protein (GSAP), which selectively increases the production and accumulation of neurotoxic beta-amyloid plaques. This suggests molecules that target at GSAP and are able to cross blood-brain barrier are potential therapeutic agents for treating Alzheimer's disease. Another study suggests that imatinib may not need to cross the blood-brain barrier to be effective at treating Alzheimer's, as the research indicates the production of beta-amyloid may begin in the liver. Tests on mice indicate that imiatinib is effective at reducing beta-amyloid in the brain. It is not known whether reduction of beta-amyloid is a feasible way of treating Alzheimer's, as an anti-beta-amyloid vaccine has been shown to clear the brain of plaques without having any effect on Alzheimer symptoms.
Adverse effects

The most common side effects include weight gain, reduced number of blood cells (neutropenia, thrombocytopenia, anemia), headache, edema, nausea, rash, and musculoskeletal pain.
Severe congestive cardiac failure is an uncommon but recognized side effect of imatinib and mice treated with large doses of imatinib show toxic damage to their myocardium.
If imatinib is used in prepubescent children, it can delay normal growth, although a proportion will experience catch-up growth during puberty.
Pharmacology
Pharmacokinetics
Imatinib is rapidly absorbed when given by mouth, and is highly bioavailable: 98% of an oral dose reaches the bloodstream. Metabolism of imatinib occurs in the liver and is mediated by several isozymes of the cytochrome P450 system, including CYP3A4 and, to a lesser extent, CYP1A2, CYP2D6, CYP2C9, and CYP2C19. The main metabolite, N-demethylated piperazine derivative, is also active. The major route of elimination is in the bile and feces; only a small portion of the drug is excreted in the urine. Most of imatinib is eliminated as metabolites, only 25% is eliminated unchanged. The half-lives of imatinib and its main metabolite are 18 and 40 hours, respectively. It blocks the activity of Abelson cytoplasmic tyrosine kinase (ABL), c-Kit and the platelet-derived growth factor receptor (PDGFR). As an inhibitor of PDGFR, imatinib mesylate appears to have utility in the treatment of a variety of dermatological diseases. Imatinib has been reported to be an effective treatment for FIP1L1-PDGFRalpha+ mast cell disease, hypereosinophilic syndrome, and dermatofibrosarcoma protuberans.
Mechanism of action
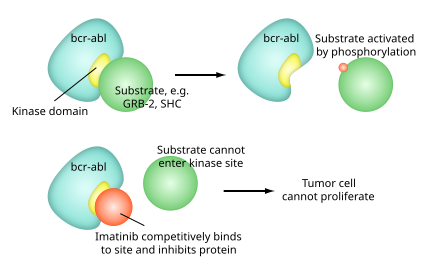
Imatinib is a 2-phenylaminopyrimidine derivative that functions as a specific inhibitor of a number of tyrosine kinase enzymes. It occupies the TK active site, leading to a decrease in activity.
There are a large number of TK enzymes in the body, including the insulin receptor. Imatinib is specific for the TK domain in abl (the Abelson proto-oncogene), c-kit and PDGF-R (platelet-derived growth factor receptor).
In chronic myelogenous leukemia, the Philadelphia chromosome leads to a fusion protein of abl with bcr (breakpoint cluster region), termed bcr-abl. As this is now a constitutively active tyrosine kinase, imatinib is used to decrease bcr-abl activity.
The active sites of tyrosine kinases each have a binding site for ATP. The enzymatic activity catalyzed by a tyrosine kinase is the transfer of the terminal phosphate from ATP to tyrosine residues on its substrates, a process known as protein tyrosine phosphorylation. Imatinib works by binding close to the ATP binding site of bcr-abl, locking it in a closed or self-inhibited conformation, and therefore inhibiting the enzyme activity of the protein semi-competitively. This fact explains why many BCR-ABL mutations can cause resistance to imatinib by shifting its equilibrium toward the open or active conformation.
Imatinib is quite selective for bcr-abl – it does also inhibit other targets mentioned above (c-kit and PDGF-R), but no other known tyrosine kinases. Imatinib also inhibits the abl protein of non-cancer cells but cells normally have additional redundant tyrosine kinases which allow them to continue to function even if abl tyrosine kinase is inhibited. Some tumor cells, however, have a dependence on bcr-abl. Inhibition of the bcr-abl tyrosine kinase also stimulates its entry in to the nucleus, where it is unable to perform any of its normal anti-apoptopic functions.
Interactions
Since imatinib is mainly metabolised via the liver enzyme CYP3A4, substances influencing the activity of this enzyme change the plasma concentration of the drug. An example of a drug that increases imatinib activity and therefore side effects by blocking CYP3A4 is ketoconazole. The same could be true of itraconazole, clarithromycin, grapefruit juice, among others. Conversely, CYP3A4 inductors like rifampicin and St. John's Wort reduce the drug's activity, risking therapy failure. Imatinib also acts as an inhibitor of CYP3A4, 2C9 and 2D6, increasing the plasma concentrations of a number of other drugs like simvastatin, ciclosporin, pimozide, warfarin, metoprolol, and possibly paracetamol. The drug also reduces plasma levels of levothyroxin via an unknown mechanism.
As with other immunosuppressants, application of live vaccines is contraindicated because the microorganisms in the vaccine could multiply and infect the patient. Inactivated and toxoid vaccines do not hold this risk, but may not be effective under imatinib therapy.
Costs

The cost of Gleevec for CML is $32,000 to $98,000 a year, and for GIST is $64,800 a year.
Prices for a 100 mg pill of Gleevec internationally range from $20 to $30, although generic imatinib is cheaper.
Legal challenge to generics
In 2007, imatinib became a test case through which Novartis challenged India's patent laws. A win for Novartis would make it harder for Indian companies to produce generic versions of drugs still manufactured under patent elsewhere in the world. Doctors without Borders argues a change in law would make it impossible for Indian companies to produce cheap generic antiretrovirals (anti-HIV medication), thus making it impossible for Third World countries to buy these essential medicines. On 6 August 2007, the Madras High Court dismissed the writ petition filed by Novartis challenging the constitutionality of Section 3(d) of Indian Patent Act, and deferred to the World Trade Organization (WTO) forum to resolve the TRIPS compliance question. As of 2008 the case is unresolved .
See also
References
- ^ A Conversation With Brian J. Druker, M.D., Researcher Behind the Drug Gleevec by Claudia Dreifus, The New York Times, November 2, 2009
- Gambacorti-Passerini C (2008). "Part I: Milestones in personalised medicine--imatinib". Lancet Oncology. 9 (600): 600. doi:10.1016/S1470-2045(08)70152-9. PMID 18510992.
- Druker BJ, Lydon NB (2000). "Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia". J. Clin. Invest. 105 (1): 3–7. doi:10.1172/JCI9083. PMC 382593. PMID 10619854.
{{cite journal}}: Unknown parameter|month=ignored (help) - Droogendijk HJ, Kluin-Nelemans HJ, van Doormaal JJ, Oranje AP, van de Loosdrecht AA, van Daele PL (2006). "Imatinib mesylate in the treatment of systemic mastocytosis: a phase II trial". Cancer. 107 (2): 345–51. doi:10.1002/cncr.21996. PMID 16779792.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Deininger MW, Druker BJ (2003). "Specific targeted therapy of chronic myelogenous leukemia with imatinib". Pharmacol. Rev. 55 (3): 401–23. doi:10.1124/pr.55.3.4. PMID 12869662.
{{cite journal}}: Unknown parameter|month=ignored (help) - Yang, FC; Ingram, DA; Chen, S; Zhu, Y; Yuan, J; Li, X; Yang, X; Knowles, S; Horn, W (2008). "Nf1-dependent tumors require a microenvironment containing Nf1+/-- and c-kit-dependent bone marrow". Cell. 135 (3): 437–48. doi:10.1016/j.cell.2008.08.041. PMC 2788814. PMID 18984156.
- "Gleevec Holds Potential As First Drug To Successfully Treat Neurofibromatosis, Scientists Report", Science Daily, October 31, 2008
- "Gleevec NF1 Trial"
- "GIST in Neurofibromatosis 1"
- "Pilot Study of Gleevec/Imatinib Mesylate (STI-571, NSC 716051) in Neurofibromatosis (NF1) Patient With Plexiform Neurofibromas (0908-09)" (Suspended)
- Tapper EB, Knowles D, Heffron T, Lawrence EC, Csete M (2009). "Portopulmonary hypertension: imatinib as a novel treatment and the Emory experience with this condition". Transplant. Proc. 41 (5): 1969–71. doi:10.1016/j.transproceed.2009.02.100. PMID 19545770.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J (2003). "LRP: role in vascular wall integrity and protection from atherosclerosis". Science. 300 (5617): 329–32. doi:10.1126/science.1082095. PMID 12690199.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Lassila M, Allen TJ, Cao Z; et al. (2004). "Imatinib attenuates diabetes-associated atherosclerosis". Arterioscler. Thromb. Vasc. Biol. 24 (5): 935–42. doi:10.1161/01.ATV.0000124105.39900.db. PMID 14988091.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Reeves PM, Bommarius B, Lebeis S; et al. (2005). "Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases". Nat. Med. 11 (7): 731–9. doi:10.1038/nm1265. PMID 15980865.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - He G, Luo W, Li P; et al. (2010). "Gamma-secretase activating protein is a therapeutic target for Alzheimer's disease". Nature. 467 (7311): 95–8. doi:10.1038/nature09325. PMC 2936959. PMID 20811458.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - http://www.msnbc.msn.com/id/41971124/ns/health-alzheimers_disease/
-
Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JAR (2008). "Long-term effects of Aβ42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial". The Lancet. 372 (9634): 216–233. doi:10.1016/S0140-6736(08)61075-2. PMID 18640458.
{{cite journal}}:|access-date=requires|url=(help);|format=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 3-85200-196-X.
- Kerkelä R, Grazette L, Yacobi R; et al. (2006). "Cardiotoxicity of the cancer therapeutic agent imatinib mesylate". Nat. Med. 12 (8): 908–16. doi:10.1038/nm1446. PMID 16862153.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Shima H, Tokuyama M, Tanizawa A; et al. (2011). "Distinct impact of imatinib on growth at prepubertal and pubertal ages of children with chronic myeloid leukemia". J. Pediatr (Online). doi:10.1016/j.jpeds.2011.03.046. PMID 21592517.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Scheinfeld N, Schienfeld N (2006). "A comprehensive review of imatinib mesylate (Gleevec) for dermatological diseases". J Drugs Dermatol. 5 (2): 117–22. PMID 16485879.
{{cite journal}}: Unknown parameter|month=ignored (help) - Takimoto CH, Calvo E. "Principles of Oncologic Pharmacotherapy" in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach. 11 ed. 2008.
- Gambacorti-Passerini CB, Gunby RH, Piazza R, Galietta A, Rostagno R, Scapozza L (2003). "Molecular mechanisms of resistance to imatinib in Philadelphia-chromosome-positive leukaemias". Lancet Oncol. 4 (2): 75–85. doi:10.1016/S1470-2045(03)00979-3. PMID 12573349.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Vigneri P, Wang JY (2001). "Induction of apoptosis in chronic myelogenous leukemia cells through nuclear entrapment of BCR-ABL tyrosine kinase". Nat. Med. 7 (2): 228–34. doi:10.1038/84683. PMID 11175855.
{{cite journal}}: Unknown parameter|month=ignored (help) - Klopp, T, ed. (2010). Arzneimittel-Interaktionen (in German) (2010/2011 ed.). Arbeitsgemeinschaft für Pharmazeutische Information. ISBN 978-3-85200-207-1.
- Schiffer CA (2007). "BCR-ABL tyrosine kinase inhibitors for chronic myelogenous leukemia". N. Engl. J. Med. 357 (3): 258–65. doi:10.1056/NEJMct071828. PMID 17634461.
{{cite journal}}: Unknown parameter|month=ignored (help) - As Pills Treat Cancer, Insurance Lags Behind, By ANDREW POLLACK, New York Times, April 14, 2009
- Living With a Formerly fatal Blood Cancer, By JANE E. BRODY, New York Times, January 18, 2010
- Kelley RK, Venook AP (2010). "Nonadherence to imatinib during an economic downturn". N. Engl. J. Med. 363 (6): 596–8. doi:10.1056/NEJMc1004656. PMID 20818898.
{{cite journal}}: Unknown parameter|month=ignored (help) - Patented Medicine Review Board (Canada). Report on New Patented Drugs - Gleevec.
- pharmacychecker.com
- Médecins Sans Frontières. "As Novartis Challenges India's Patent Law, MSF Warns Access to Medicines Is Under Threat", 2006-09-26. Accessed 2006-02-10.
External links
| Piperazines | |
|---|---|
| Simple piperazines (no additional rings) | |
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines | |
| Pyridinylpiperazines | |
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized | |