This is an old revision of this page, as edited by Beetstra (talk | contribs) at 20:35, 10 November 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank', 'ChEMBL').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 20:35, 10 November 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank', 'ChEMBL').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound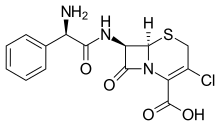 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682729 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well absorbed, independent of food intake |
| Metabolism | 15% to 40% |
| Elimination half-life | 0.6 to 0.9 hours |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.536 |
| Chemical and physical data | |
| Formula | C15H14ClN3O4S |
| Molar mass | 367.808 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Cefaclor, also known as cefachlor or cefaclorum (brand names Ceclor, Distaclor, Keflor, Raniclor), is a second-generation cephalosporin antibiotic used to treat certain infections caused by bacteria such as pneumonia and ear, lung, skin, throat, and urinary tract infections.
Indications
Cefaclor belongs to the family of antibiotics known as the cephalosporins (cefalosporins). The cephalosporins are broad-spectrum antibiotics that are used for the treatment of septicaemia, pneumonia, meningitis, biliary-tract infections, peritonitis, and urinary-tract infections. The pharmacology of the cephalosporins is similar to that of the penicillins, excretion being principally renal. Cephalosporins penetrate the cerebrospinal fluid poorly unless the meninges are inflamed; cefotaxime is a more suitable cephalosporin than cefaclor for infections of the central nervous system, e.g. meningitis. Cefaclor is active against many bacteria, including both Gram-negative and Gram-positive organisms.
Cautions and contraindications
Cautions include known sensitivity to beta-lactam antibacterials, such as penicillins (Cefaclor should be avoided if there is a history of immediate hypersensitivity reaction); renal impairment (no dose adjustment required, although manufacturer advises caution); pregnancy and breast-feeding (but appropriate to use); false positive urinary glucose (if tested for reducing substances) and false positive Coombs test. Cefaclor has also been reported to cause a serum sickness-like reaction in children.
Cefaclor is contraindicated in case of hypersensitivity (i.e. allergy) to cephalosporins.
Side effects
The principal side effect of the cephalosporins is hypersensitivity. Up to about 10% of penicillin-sensitive patients will also be allergic to the cephalosporins, depending on the cephalosporin generation. Allergic reactions may present as, for example, rashes, pruritus (itching), urticaria, serum sickness-like reactions with rashes, fever and arthralgia, and anaphylaxis. The frequency and severity of serum sickness-like reactions in children has led researchers to question its role in pediatric illness.

Other side effects include gastrointestinal disturbances (e.g. diarrhea, nausea and vomiting, abdominal discomfort, disturbances in liver enzymes, transient hepatitis and cholestatic jaundice), headache, and Stevens-Johnson syndrome. Rare side effects include eosinophilia and blood disorders (including thrombocytopenia, leucopenia, agranulocytosis, aplastic anaemia and haemolytic anaemia); reversible interstitial nephritis; hyperactivity, nervousness, sleep disturbances, hallucinations, confusion, hypertonia, and dizziness. Toxic epidermal necrolysis has been reported. In the UK, The Committee on the Safety of Medicines (CSM) has warned that the risk of diarrhea and rarely antibiotic-associated colitis are more likely with higher doses.
Interactions with other medications
Coumarins
Cephalosporins possibly enhance the anticoagulant effect of coumarins (e.g. Warfarin) - change in patient's clinical condition, particularly associated with liver disease, intercurrent illness, or drug administration, necessitates more frequent testing of INR, and dose adjustment as necessary.
Probenecid
Excretion of cephalosporins is reduced by probenecid (resulting in increased concentrations of drug in the blood plasma).
Antacids
Absorption of cefaclor is reduced by H2 blockers (a type of antacid); therefore antacids should not be taken at the same time as cefaclor.
Safety in pregnancy and breastfeeding
Cefaclor is passed into the breast milk in small quantities, but is generally accepted to be safe to take during breastfeeding. Cefaclor is not known to be harmful in pregnancy.
Cefaclor CD
Cefaclor CD is a sustained release form of Cefaclor which releases the drug to the body over a longer period of time, which means that doses can be taken less frequently, with steadier levels of the drug in the bloodstream. Sustained release is useful with Cefaclor as it has a very short half-life.
References
- Hebert A, Sigman E, Levy M (1991). "Serum sickness-like reactions from cefaclor in children". J Am Acad Dermatol. 25 (5 Pt 1): 805–8. doi:10.1016/S0190-9622(08)80973-5. PMID 1802903.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Parra F, Igea J, Martín J, Alonso M, Lezaun A, Sainz T (1992). "Serum sickness-like syndrome associated with cefaclor therapy". Allergy. 47 (4 Pt 2): 439–40. doi:10.1111/j.1398-9995.1992.tb02086.x. PMID 1456417.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - King BA, Geelhoed GC (2003). "Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions". J Paediatr Child Health. 39 (9): 677–81. doi:10.1046/j.1440-1754.2003.00267.x. PMID 14629499.
{{cite journal}}: Unknown parameter|month=ignored (help) - LactMED. "Summary of Cefaclor's use during lactation". National Library of Medicine. Retrieved 22 May 2011.
- Ito, S. (1993). "Prospective follow-up of adverse reactions in breast-fed infants exposed to maternal medication". Am J Obstet Gynecol. 168 (168): 1393–1399. PMID 8498418.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)