This is an old revision of this page, as edited by Beetstra (talk | contribs) at 10:50, 11 November 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEMBL').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 10:50, 11 November 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEMBL').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
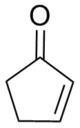
| IUPAC name 2-Cyclopenten-1-one | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.012.012 | ||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C5H6O | ||
| Molar mass | 82.04 g·mol | ||
| Density | 0.98 g·mL | ||
| Boiling point | 150 °C | ||
| Solubility in water | almost insoluble in water | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Harmful | ||
| Flash point | 42 °C | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Cyclopentenone is a hydrocarbon with chemical formula Template:Carbon5Template:Hydrogen6Template:Oxygen and CAS number 930-30-3. It is structurally similar to cyclopentanone, with the additional feature of α-β unsaturation in the ring system. Cyclopentenone belongs to the cycloalkene (alkenes which have one or more rings of carbon atoms) class of compounds and is also a ketone (it possesses a carbonyl functional group). It is a liquid at room temperature with a boiling point of 150 °C. It has been isolated from pressure-cooked pork liver by simultaneous steam distillation and continuous solvent extraction.
The term cyclopentenone may also refer to a structural motif wherein the cyclopentenone moiety is a subsection of a larger molecule. In this regard, cyclopentenones are found in a large number of natural products, including jasmone, the aflatoxins, and several prostaglandins.
Synthesis
Cyclopentenones can be synthesized in a number of ways. Industrially, the most common procedures are elimination of α-bromo-cyclopentanone using lithium carbonate and Claisen condensation-decarboxylation-isomerization cascades of unsaturated diesters as shown below.

As a functional group, the synthesis of cyclopentenones is accomplished in a variety of other ways, including the Nazarov cyclization reaction from divinyl ketones, Saegusa–Ito oxidation from cyclopentanones, ring-closing metathesis from the corresponding dienes, oxidation of the corresponding cyclic allylic alcohols, and the Pauson–Khand reaction from alkenes, alkynes, and carbon monoxide.
Reactions
As an enone, cyclopentenone undergoes the typical reactions of α-β unsaturated ketones, including nucleophilic conjugate addition, the Baylis–Hillman reaction, and the Michael reaction. Cyclopentenone also functions as an excellent dienophile in the Diels–Alder reaction, reacting with a wide variety of dienes. In one example, a Danishefsky-type diene is reacted with a cyclopentenone to yield a fused tricyclic system en route to the synthesis of coriolin.

References
- Mussinan,C.J.; Walradt, J.P. (1974), J. Agric. Food Chem., 22 (5): 827–831, doi:10.1021/jf60195a002
{{citation}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - Process for producing 2-bromocyclopentanone, 2004-05-12
{{citation}}: Unknown parameter|country-code=ignored (help); Unknown parameter|inventor-first=ignored (help); Unknown parameter|inventor-last=ignored (help); Unknown parameter|inventor2-first=ignored (help); Unknown parameter|inventor2-last=ignored (help); Unknown parameter|patent-number=ignored (help) - Process for preparing cyclopentenone, 2004-11-25
{{citation}}: Unknown parameter|country-code=ignored (help); Unknown parameter|inventor-first=ignored (help); Unknown parameter|inventor-last=ignored (help); Unknown parameter|inventor2-first=ignored (help); Unknown parameter|inventor2-last=ignored (help); Unknown parameter|inventor3-first=ignored (help); Unknown parameter|inventor3-last=ignored (help); Unknown parameter|inventor4-first=ignored (help); Unknown parameter|inventor4-last=ignored (help); Unknown parameter|patent-number=ignored (help) - Organic Reactions Portal "Cyclopentenone synthesis"
- Danishefsky, S.; Zamboni, R.; Kahn, M.; Etheredge, S.J. (1980), J. Am. Chem. Soc., 102 (6): 2097–2098, doi:10.1021/ja00526a061
{{citation}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link)