This is an old revision of this page, as edited by Beetstra (talk | contribs) at 12:25, 23 November 2011 (Saving copy of the {{drugbox}} taken from revid 456658868 of page Lubiprostone for the Chem/Drugbox validation project (updated: 'DrugBank', 'ChEMBL').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:25, 23 November 2011 by Beetstra (talk | contribs) (Saving copy of the {{drugbox}} taken from revid 456658868 of page Lubiprostone for the Chem/Drugbox validation project (updated: 'DrugBank', 'ChEMBL').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{drugbox}}) taken from revid 456658868 of page Lubiprostone with values updated to verified values. |
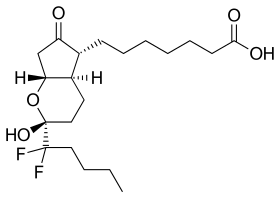 | |
| Clinical data | |
|---|---|
| Trade names | Amitiza |
| Other names | Amitiza RU-0211 SPI-0211 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607034 |
| License data |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Negligible |
| Protein binding | 94% |
| Metabolism | Extensive, CYP not involved |
| Elimination half-life | Unknown (lubiprostone) 0.9–1.4 hours (main metabolite) |
| Excretion | Renal (60%) and fecal (30%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C20H32F2O5 |
| Molar mass | 390.462 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |