This is an old revision of this page, as edited by Lamro (talk | contribs) at 09:06, 24 November 2011 (link). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 09:06, 24 November 2011 by Lamro (talk | contribs) (link)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |
| Identifiers | |
|---|---|
| 3D model (JSmol) | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C19H15 |
| Molar mass | 243.329 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
The triphenylmethyl radical is a persistent radical and the first-ever radical described in organic chemistry. It can be prepared by homolysis of triphenylmethyl chloride 1 (scheme 1) by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid type dimer 3. In benzene the concentration of the radical is 2% .
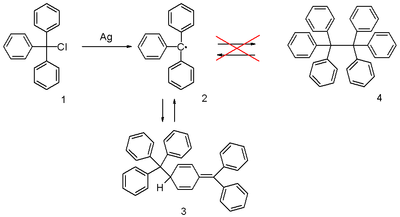
Solutions containing the radical are yellow and when the temperature of the solution is increased the yellow color becomes more intense as the equilibrium is shifted in favor of the radical following Le Chatelier's principle. Conversely when the solution is cooled it becomes less yellow.
When exposed to air the radical rapidly oxidizes to the peroxide (Scheme 2) and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with iodine to triphenylmethyl iodide.
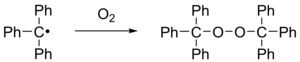
The radical was discovered by Moses Gomberg in 1900. He tried to prepare hexaphenylethane from triphenylmethyl chloride and zinc in benzene in a Wurtz reaction and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated.
The correct quinoid structure for the dimer was suggested as early as 1904 but this structure was soon after abandoned by the scientific community in favor of hexaphenylethane which is structure 4 in scheme 1 . It subsequently took until 1968 for its rediscovery when researchers at the Vrije Universiteit Amsterdam published proton NMR data . In hindsight the substituted ethane molecule does not make sense at all because it is simply too sterically overcrowded.
Miscellany
Gomberg concluded his 1900 article with the sentence "This work will be continued and I wish to reserve the field for myself." He ended his 1901 article by writing, "It is my intention to extend this study to other oxygen compounds, as well as to nitrogen derivatives, and I beg to reserve this field for further work." It is true that nineteenth-century chemists did not intrude on each other's research; to his dismay, Gomberg found out that this was not the case in the twentieth century.
References
- Advanced Organic Chemistry J. March, John Wiley & Sons ISBN 0-471-88841-9
- M. Gomberg (1900). "An instance of trivalent carbon: triphenylmethyl". J. Am. Chem. Soc. 22 (11): 757–771. doi:10.1021/ja02049a006.
- M. Gomberg (1901). "On trivalent carbon". J. Am. Chem. Soc. 23 (7): 496–502. doi:10.1021/ja02033a015. (Note: radical is also called a cadicle)
- M. Gomberg (1902). "On trivalent carbon". J. Am. Chem. Soc. 24 (7): 597–628. doi:10.1021/ja02021a001.
- J. M. McBride (1974). "The hexaphenylethane riddle". Tetrahedron. 30 (14): 2009–2022. doi:10.1016/S0040-4020(01)97332-6.
- H. Lankamp, W. Th. Nauta and C. MacLean (1968). "A new interpretation of the monomer-dimer equilibrium of triphenylmethyl- and alkylsubstituted-diphenyl methyl-radicals in solution". Tetrahedron Letters. 9 (2): 249–254. doi:10.1016/S0040-4039(00)75598-5.
External links
- Molecule of the Month June 1997 Link
- Experimental procedures Link