This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 02:45, 11 December 2011 (Updating {{chembox}} (changes to watched fields - updated 'DrugBank_Ref', 'UNII_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or bugs)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 02:45, 11 December 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (changes to watched fields - updated 'DrugBank_Ref', 'UNII_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or bugs))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)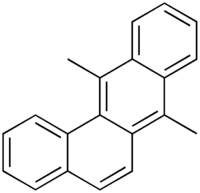
| |

| |
| Names | |
|---|---|
| IUPAC name 7,12-dimethylbenzophenanthrene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.326 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H16 |
| Molar mass | 256.34104 |
| Melting point | 122–123 °C |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | T (Toxic) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
7,12-Dimethylbenz(a)anthracene is an immunosuppressor and a powerful organ-specific laboratory carcinogen. Also known as 7,12-dimethylbenzanthracene or DMBA, this substance is widely used in many research laboratories studying cancer. DMBA serves as a tumor initiator by making necessary mutations. Tumor promotion can be induced with treatments of TPA (12-O-tetradecanoylphorbol-13-acetate) in some models of two-stage carcinogenesis. This allows for a greatly accelerated rate of tumor growth, making many cancer studies possible.
References
- 7,12-Dimethylbenz(a)anthracene at Sigma-Aldrich
- Miyata M, Furukawa M, Takahashi K, Gonzalez FJ, Yamazoe Y. (2001) Mechanism of 7, 12-Dimethylbenzanthracene-Induced Immunotoxicity: Role of Metabolic Activation at the Target Organ. Jpn J Pharmacol 86:302-309. Link
- Sung YM, He G, Fischer, SM. (2005) Lack of Expression of the EP2 but not EP3 Receptor for Prostaglandin E2 Results in Suppression of Skin Tumor Development. Cancer Res 65:9304-9311