This is an old revision of this page, as edited by Wtshymanski (talk | contribs) at 15:02, 15 December 2011 (expand initialisms). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:02, 15 December 2011 by Wtshymanski (talk | contribs) (expand initialisms)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)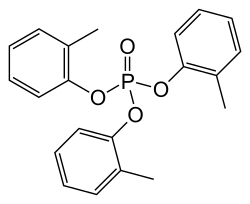 | |
| Names | |
|---|---|
| Other names tricresylphosphate, tri-o-cresyl phosphate, TOCP, tritolyl phosphate, tolyl phosphate, tri-o-tolyl ester of phosphoric acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.014.136 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H21O4P |
| Molar mass | 368.37 g/mole |
| Appearance | colourless liquid |
| Melting point | -40 °C |
| Boiling point | 255 °C (10mm Hg) |
| Hazards | |
| Flash point | > 225 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tricresyl phosphate, abbreviated TCP, is an organophosphate compound that is used as a plasticizer and diverse other applications. It is a colourless, viscous liquid, although commercial samples are typically yellow. It is virtually insoluble in water.
Production
Tricresyl phosphate is manufactured by reaction of cresols with phosphorus oxychloride:
- OPCl3 + 3 HOC6H4CH3 → OP(OC6H4CH3)3 + 3 HCl
The cresol is a mixture of three isomers (ortho, meta, and para). The fact that tricresyl phosphate is derived from a mixture and itself is a mixture ensures that it remains liquid over a wide span of temperatures.
Chemical reactions
In alkaline medium it undergoes hydrolysis to cresol and dicresyl phosphate.
- OP(OC6H4CH3)3 + NaOH → + HOC6H4CH3 + NaO2P(OC6H4CH3)2
In the body, it is metabolized in part by hydroxylation to give a catecholate derivative, which is the bio-active agent responsible for the neurotoxicity.
Uses
Tricresyl phosphate is used as a plasticizer in nitrocellulose, acrylate lacquers, varnishes, and in polyvinyl chloride. It is a flame retardant in plastics and rubbers. It is used as a gasoline additive as a lead scavenger for tetra-ethyl lead. It is a hydraulic fluid and a heat exchange medium. Exploiting its hydrophobic properties, it is used for the waterproofing of materials. It is a solvent for extractions, a solvent for nitrocellulose and other polymers. It is used as an antiwear and extreme pressure additive in lubricants and hydraulic fluids.
Safety
TCP is the cause of numerous poisonings and is a neurotoxin, in part via organophosphate-induced delayed neuropathy. It is of “toxicological importance” and has been responsible for many deaths. One of the most serious incidents occurred in the 1920s when TCP was used as an adulterant for Jamaica Ginger. Another occurred in Morocco, in 1959, when cooking oil was adulterated with jet engine lubricant containing TCP.
TCP's mechanism of action is similar to other organophosphates in that it can inhibit the enzyme acetylcholinesterase, leading to a buildup of acetylcholine in the synaptic space. This can lead to hyperactivity in cholinergic neurons in the brain and at neuromuscular junctions in the peripheral nervous system resulting in apoptosis of those cell-types. This is the reason for paralysis and other irreversible neurological problems seen in the "Gingerjake" syndromes during prohibition, when TCP was added to gingerjake moonshine.
TCP is used as an additive in turbine engine oil and can potentially get into the airliner cabins via a bleed air "fume event". Aerotoxic syndrome is the name given to the alleged health ill-effects caused by exposure to engine chemicals; UK industry-funded studies are yet to make a link between TCP and any long-term health issues.
TCP is combustible, although less so than typical organic compounds.
References
- ^ Jürgen Svara, Norbert Weferling, Thomas Hofmann "Phosphorus Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH: Wienheim. doi:10.1002/14356007.a19_545.pub2
- Marvel & other solutions to the lead problem, checked 2009-06-18.
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.chroma.2007.05.087, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.chroma.2007.05.087instead. - Lack of Delayed Neurotoxic Effect after Tri-o-cresyl Phosphate Treatment in Male Fischer 344 Rats: Biochemical, Neurobehavioral and Neuropathological Studies
- Segalla, Spencer (2011). "The 1959 Moroccan Oil Poisoning and US Cold War Disaster Diplomacy." Journal of North African Studies. Available online at http://dx.doi.org/10.1080/13629387.2011.610118
- UK Parliament: Elements of healthy cabin air