This is an old revision of this page, as edited by Kilom691 (talk | contribs) at 13:05, 12 February 2012 (ref corrected). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:05, 12 February 2012 by Kilom691 (talk | contribs) (ref corrected)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)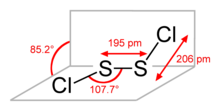
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Disulfur dichloride | |||
| Systematic IUPAC name Dichlorodisulfane | |||
| Other names
Bis(S–S) Dimeric sulfenic chloride | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.030.021 | ||
| EC Number |
| ||
| MeSH | Sulfur+monochloride | ||
| PubChem CID | |||
| RTECS number |
| ||
| UN number | 3390 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | S2Cl2 | ||
| Molar mass | 135.04 g/mol | ||
| Appearance | yellow liquid | ||
| Density | 1.688 g/cm | ||
| Melting point | −80 °C (−112 °F; 193 K) | ||
| Boiling point | 137.1 °C (278.8 °F; 410.2 K) | ||
| Solubility in water | decomp with loss of HCl | ||
| Solubility | soluble in ethanol, benzene, ether, chloroform, CCl4 | ||
| Refractive index (nD) | 1.658 | ||
| Structure | |||
| Coordination geometry | gauche | ||
| Dipole moment | 1.60 D | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 118.5 °C | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Disulfur dichloride is the chemical compound with the formula S2Cl2 . Some alternative names for this compound are sulfur monochloride (the name implied by its empirical formula, SCl), disulphur dichloride (British English Spelling) and sulphur monochloride (British English Spelling). S2Cl2 has the structure implied by the formula Cl-S-S-Cl, wherein the angle between the Cl-S-S and S-S-Cl planes is 90°. This structure is referred to as gauche, and is akin to that for H2O2. A different isomer of S2Cl2 is S=SCl2; this isomer forms transiently when S2Cl2 is exposed to UV-radiation (see thiosulfoxides).
Synthesis and basic properties
Pure disulfur dichloride is a yellow liquid that smokes in air due to reaction with water:
- 2 S2Cl2 + 2 H2O → SO2 + 4 HCl + 3/8 S8
It is synthesized by partial chlorination of elemental sulfur. The reaction takes place at usable rates at room temperature. In the laboratory, chlorine gas is led into a flask containing elemental sulfur. As disulfur dichloride is formed, the contents become a golden yellow liquid:
- S8 + 4 Cl2 → 4 S2Cl2 ΔH = −58.2 kJ/mol
Excess chlorine gives sulfur dichloride which causes the liquid to become less yellow and more orange-red:
- S2Cl2 + Cl2 ↔ 2 SCl2 ΔH = −40.6 kJ/mol
The reaction is reversible, and upon standing, SCl2 releases chlorine to revert to the disulfur dichloride. Disulfur dichloride has the ability to dissolve large quantities of sulfur, which reflects in part the formation of polysulfanes:
- S2Cl2 + n S → S2+nCl2
Pure disulfur dichloride is obtained by distilling the yellow-orange liquid over excess elemental sulfur.
S2Cl2 also arises from the chlorination of CS2 as in the synthesis of thiophosgene.
Applications
S2Cl2 has been used to introduce C-S bonds. In the presence of AlCl3, S2Cl2 reacts with benzene to give diphenyl sulfide:
- S2Cl2 + 2 C6H6 → (C6H5)2S + 2 HCl + 1/8 S8
Anilines react with S2Cl2 in the presence of NaOH via the so-called Herz reaction to give ortho-aminothiophenolates. These species are precursors to thioindigo dyes. It is also used to prepare the sulfur mustard "gas" by reaction with ethylene at 60°C (the Levinstein process):
- S2Cl2 + 2 C2H4 → (ClC2H4)2S + 1/8 S8
- S2Cl2 + 2 C2H4 → (ClC2H4)2S + 1/8 S8
Other uses include manufacturing sulfur dyes, insecticides, synthetic rubbers. Also used in cold vulcanization of rubbers, as polymerization catalyst for vegetable oils and for hardening soft woods.
References
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Hartman, W. W.; Smith, L. A.; Dickey, J. B. (1934). "Diphenylsulfide". Organic Syntheses. 14: 36; Collected Volumes, vol. 2, p. 242.
- R. J. Cremlyn “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). ISBN 0-471-95512-4
- Garcia-Valverde M., Torroba T. (2006). "Heterocyclic chemistry of sulfur chlorides - Fast ways to complex heterocycles". European Journal of Organic Chemistry. 4 (4): 849–861. doi:10.1002/ejoc.200500786.
- F. Fehér "Dichlorodisulfane" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 371.

