This is an old revision of this page, as edited by Vuo (talk | contribs) at 18:04, 3 March 2012 (→Organic chemistry: subsection separator serves no purpose). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 18:04, 3 March 2012 by Vuo (talk | contribs) (→Organic chemistry: subsection separator serves no purpose)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) For other uses, see Dimer (disambiguation).| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Dimerization" – news · newspapers · books · scholar · JSTOR (April 2009) (Learn how and when to remove this message) |

A dimer is a chemical entity consisting of two structurally similar monomers joined by bonds that can be either strong or weak.
Molecular dimers are often formed by the reaction of two identical compounds e.g.: 2A → A-A. In this example, monomer "A" is said to dimerise to give the dimer "A-A". An example is a diaminocarbene, which dimerise to give a tetraaminoethylene:
- 2 C(NR2)2 → (R2N)2C=C(NR2)2
Acetic acid forms a dimer in the gas phase, the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer.
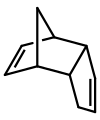
Dicyclopentadiene is an asymmetrical dimer of two cyclopentadiene molecules that have reacted in a Diels-Alder reaction to give the product. Upon heating, it "cracks" (undergoes a retro-Diels-Alder reaction) to give identical monomers:
- C10H12 → 2 C5H6
The term homodimer is used when the two molecules are identical (e.g. A-A) and heterodimer when they are not (e.g. A-B). The reverse of dimerisation is often called dissociation.
See also
References
- "IUPAC "Gold Book" definition". Retrieved 2009-04-30.