This is an old revision of this page, as edited by 170.200.144.6 (talk) at 18:54, 1 July 2013. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 18:54, 1 July 2013 by 170.200.144.6 (talk)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| Some of this article's listed sources may not be reliable. Please help improve this article by looking for better, more reliable sources. Unreliable citations may be challenged and removed. (June 2012) (Learn how and when to remove this message) |
 | |
 | |
| Combination of | |
|---|---|
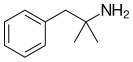
| Phentermine | Appetite suppressant/stimulant of the amphetamine and phenethylamine class |
| Topiramate | Anticonvulsant |
| Clinical data | |
| Trade names | Qsymia |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| (verify) | |
The combination of the drugs phentermine and topiramate (trade name Qsymia, formerly Qnexa) is a medication for the treatment of obesity and potentially related conditions such as type 2 diabetes and has been found to lower blood pressure and cholesterol. Qsymia was developed by Vivus, a California pharmaceutical company. Phentermine is an appetite suppressant and stimulant of the amphetamine and phenethylamine class. Topiramate is an anticonvulsant that has weight loss side effects.
On February 22, 2012, U.S. Food and Drug Administration (FDA) advisors voted 20 to 2 to recommend that the FDA adopt phentermine/topiramate as an obesity treatment. Final approval was expected later in 2012, with recommendations for post-market monitoring for cardiovascular risk and an indication against use by pregnant women. On July 17, 2012, the U.S. FDA approved Qsymia as an addition to a reduced-calorie diet and exercise for chronic weight management,for thoses with a BMI of at least 30. Qsymia is available in certified retail pharmacies nationwide. It is also available through a certified mail-order pharmacy network.
Safety and effectiveness
Clinical studies have shown weight loss under treatment with Qnexa. The phase 3, 56-week EQUIP study showed that the average weight loss of 14.7% (37 lbs) was achieved by obese patients treated with Qnexa. The following doses of phentermine IR and topiramate CR were used in Phase 3 testing:
- Full strength formula: 15 mg of phentermine IR (instant-release) and 92 mg of topiramate CR (controlled-release)
- Mid strength formula: 7.5 mg phentermine IR and 46 mg topiramate CR
- Low strength formula: 3.75 mg phentermine IR and 23 mg topiramate CR
In 2009, Vivus reported that the main side effects during testing phases were dry mouth, a tingling in the fingers and toes and constipation. However, in 2010 Public Citizen's Dr. Sidney M. Wolfe testified before the FDA Advisory Committee that studies showed that Qnexa carries a long list of relatively rare but potentially serious side effects, including possible birth defects
Studies and timeline
EQUIP
EQUIP investigated the combination for 56 weeks in severely obese patients.
CONQUER
A 56-week safety and efficacy trial, published in The Lancet.
SEQUEL
A 108-week trial rolling-over patients from CONQUER, collecting long-term data.
Approval history
On December 28, 2009 a new drug application (NDA) was submitted to the FDA for approval and on March 1, 2010, Vivus announced that the agency accepted the NDA.
FDA approval was declined in October 2010 due to concerns about dangerous side effects, including suicidal thoughts, heart palpitations, memory lapses and birth defects.
In January 2011, the FDA expressed concerns about the potential for Qnexa to cause birth defects and asked Vivus to examine this possibility before the drug can be approved.
Qnexa's name was changed to Qsymia and approved for sale on July 17, 2012 by the FDA.
Patents and other indications
Vivus currently has four U.S. patents covering Qnexa. These patents are related to the product and methods of using the drug in various therapeutic applications.
Qnexa is also in phase 2 clinical development for the treatment of type 2 diabetes and obstructive sleep apnea (OSA). A phase 2 safety and efficacy study evaluating Qnexa in patients with OSA showed that patients who took Qnexa had a 69 percent reduction in sleep apnea events and lost more weight than those who took placebo.
Further research data released indicates that Qnexa lowers blood pressure. Dr. Suzanne Oparil of the University of Alabama at Birmingham stated “The higher the dose, the more weight loss and the more blood pressure went down” presented at the American Society of Hypertension’s 25th annual meeting in New York. Her co-authored study was subsequently accepted and published by the American Journal of Cardiology.
References
- Vivus Inc.,
- Vivus Weight-Loss Pill is Second in Month to Win Approval
- Vivus Inc.
- ^ "FDA advisors endorse weight-loss drug Qnexa". latimes.com. 2012-02-23. Retrieved 23 February 2012.
- http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312468.htm
- http://ir.vivus.com/releasedetail.cfm?ReleaseID=774635
- Vivus Phase 3 studies find Qnexa effective in tackling obesity
- Vivus Says Qnexa, a Diet Drug, Did Well in Trials
- Weight Loss Drug Too Dangerous to Be Allowed On Market
- Controlled-Release Phentermine/Topiramate in Severely Obese Adults: A Randomized Controlled Trial (EQUIP),
- Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER).,
- Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL),
- VIVUS Submits Qnexa(R) New Drug Application to the FDA for the Treatment of Obesity
- FDA accepts Vivus application for obesity drug
- "FDA rejects second weight-loss drug in a week". msnbc.com news services. 10/29/2010 5:06:32 AM ET. Retrieved 29 October 2010.
{{cite web}}: Check date values in:|date=(help) - Vivus says FDA asks about Qnexa birth defect link, Business Week, January 21, 2011
- Hellmich, Nancy. "New diet drug helps patients lose about 10% of weight". USA Today. Retrieved 17 July 2012.
- "Medications Target Long-Term Weight Control". FDA.gov. Retrieved 17 July 2012.
- VIVUS Announces the Issuances of Three Additional Qnexa(R) Patents
- Vivus' Qnexa improves sleep apnea in small trial
- Qnexa weight loss drug lowers blood pressure: study
- Changes in Cardiovascular Risk Associated With Phentermine and Topiramate Extended-Release in Participants With Comorbidities and a Body Mass Index ≥27 kg/m2