This is an old revision of this page, as edited by Smokefoot (talk | contribs) at 14:35, 19 July 2014 (added structural formula although maybe it does not help that much, add ref and alt method). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 14:35, 19 July 2014 by Smokefoot (talk | contribs) (added structural formula although maybe it does not help that much, add ref and alt method)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)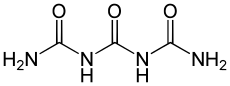
| |

| |
| Names | |
|---|---|
| Other names
Carbonyldiurea 1,3-Dicarbamylurea Dicarbamylurea Diimidotricarbonic diamide 2,4-diimidotricarbonic diamide Tricarbonodiimidic diamide | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.008.317 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | C3H6N4O3 |
| Molar mass | 146.106 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Triuret is an organic compound with the formula 2NH It is a product from the pyrolysis of urea. Triuret is a colorless, crystalline, hygroscopic solid, slightly soluble in cold water or ether, and more soluble in hot water.
Synthesis
It is prepared by heating thin layers of urea, the thin layers facilitating escape of ammonia:
- 3 2NH + 2 NH3
It can also prepared by treatment of urea with phosgene:
- 2 2NH + 2 HCl
Triuret is a complicating by-product in the industrial synthesis of melamine from urea.
Related compounds
References
- "Urea Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2012. doi:10.1002/14356007.o27_o04. ISBN 978-3527306732.
{{cite encyclopedia}}: Unknown parameter|authors=ignored (help)