This is an old revision of this page, as edited by 101.98.58.66 (talk) at 21:05, 17 February 2015 (→Religious beliefs: `1). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 21:05, 17 February 2015 by 101.98.58.66 (talk) (→Religious beliefs: `1)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) For the concept car, see Toyota Alessandro Volta.| This article's lead section may be too short to adequately summarize the key points. Please consider expanding the lead to provide an accessible overview of all important aspects of the article. (August 2012) |
| Alessandro Volta | |
|---|---|
 Alessandro Giuseppe Antonio Anastasio Volta Alessandro Giuseppe Antonio Anastasio Volta | |
| Born | 18 February 1745 Como, Duchy of Milan present-day Italy |
| Died | 5 March 1827(1827-03-05) (aged 82) Como, Lombardy-Venetia present-day Italy |
| Nationality | Italian |
| Known for | Invention of the electric cell Discovery of methane Volt Voltage Voltmeter |
| Awards | Copley Medal (1794) |
| Scientific career | |
| Fields | Physics and chemistry |
Alessandro Giuseppe Antonio Anastasio Volta (February 18, 1745 – March 5, 1827) was an Italian physicist known for the invention of the battery in the 1800s.
Early life and works
Volta was born in Como, a town in present-day northern Italy (near the Swiss border) on February 18, 1745. In 1794, Volta married an aristocratic lady also from Como, Teresa Peregrini, with whom he raised three sons: Giovanni, Flaminio and Zanino. His own father Filippo Volta was of noble lineage. His mother Donna Maddalena came from the family of the Inzaghis. In 1774, he became a professor of physics at the Royal School in Como. A year later, he improved and popularized the electrophorus, a device that produced static electricity. His promotion of it was so extensive that he is often credited with its invention, even though a machine operating on the same principle was described in 1762 by the Swedish experimenter Johan Wilcke. In 1777, he travelled through Switzerland. There he befriended H. B. de Saussure.
In the years between 1776–78, Volta studied the chemistry of gases. He researched and discovered methane after reading a paper by Benjamin Franklin of America on "flammable air". In November 1776, he found methane at Lake Maggiore, and by 1778 he managed to isolate methane. He devised experiments such as the ignition of methane by an electric spark in a closed vessel. Volta also studied what we now call electrical capacitance, developing separate means to study both electrical potential (V ) and charge (Q ), and discovering that for a given object, they are proportional. This may be called Volta's Law of capacitance, and it was for this work the unit of electrical potential has been named the volt.
In 1779 he became a professor of experimental physics at the University of Pavia, a chair that he occupied for almost 40 years.
Volta and Galvani

Luigi Galvani discovered something he named "animal electricity" when two different metals were connected in series with the frog's leg and to one another. Volta realized that the frog's leg served as both a conductor of electricity (what we would now call an electrolyte) and as a detector of electricity. He replaced the frog's leg with brine-soaked paper, and detected the flow of electricity by other means familiar to him from his previous studies. In this way he discovered the electrochemical series, and the law that the electromotive force (emf) of a galvanic cell, consisting of a pair of metal electrodes separated by electrolyte, is the difference between their two electrode potentials (thus, two identical electrodes and a common electrolyte give zero net emf). This may be called Volta's Law of the electrochemical series.
In 1800 as the result of a professional disagreement over the galvanic response advocated by Galvani, Volta invented the voltaic pile, an early electric battery, which produced a steady electric current. Volta had determined that the most effective pair of dissimilar metals to produce electricity was zinc and silver. Initially he experimented with individual cells in series, each cell being a wine goblet filled with brine into which the two dissimilar electrodes were dipped. The voltaic pile replaced the goblets with cardboard soaked in brine.
First battery
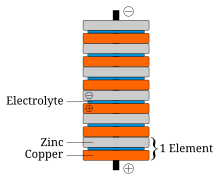
In announcing his discovery of his voltaic pile, Volta paid tribute to the influences of William Nicholson, Tiberius Cavallo, and Abraham Bennet.
The battery made by Volta is credited as the first electrochemical cell. It consists of two electrodes: one made of zinc, the other of copper. The electrolyte is either sulfuric acid mixed with water or a form of saltwater brine. The electrolyte exists in the form 2H and SO4. The zinc, which is higher than both copper and hydrogen in the electrochemical series, reacts with the negatively charged sulfate (SO4). The positively charged hydrogen ions (protons) capture electrons from the copper, forming bubbles of hydrogen gas, H2. This makes the zinc rod the negative electrode and the copper rod the positive electrode.
Thus, there are two terminals, and an electric current will flow if they are connected. The chemical reactions in this voltaic cell are as follows:
- zinc
- Zn → Zn + 2e
- sulfuric acid
- 2H + 2e → H2
The copper does not react, but rather it functions as an electrode for the electric current.
However, this cell also has some disadvantages. It is unsafe to handle, since sulfuric acid, even if diluted, can be hazardous. Also, the power of the cell diminishes over time because the hydrogen gas is not released. Instead, it accumulates on the surface of the zinc electrode and forms a barrier between the metal and the electrolyte solution.
Last years and retirement

In honor of his work, Volta was made a count by Napoleon Bonaparte in 1810. His image was depicted on the Italian 10,000 lira note (no longer in circulation, since the euro has replaced the lira) along with a sketch of his voltaic pile.
Volta retired in 1819 to his estate in Camnago, a frazione of Como, Italy, now named "Camnago Volta" in his honor. He died there on March 5, 1827. Volta's remains were buried in Camnago Volta.
Volta's legacy is celebrated by the Tempio Voltiano memorial located in the public gardens by the lake. There is also a museum which has been built in his honor, which exhibits some of the equipment that Volta used to conduct experiments. Nearby stands the Villa Olmo, which houses the Voltian Foundation, an organization promoting scientific activities. Volta carried out his experimental studies and produced his first inventions near Como.
OIUH na zjhvfcxgvhjhskdfhciusbfg gdiej tjrvfuytdf tgrfdyetdrf tluewrgfiuytwafcg87dtfuegiytknf fyugje gf jhggh hriu h kgvihfigrkfytilegr7d6rqgfudtRGFYK YT HGIUYDTIYhhgiaWegdiutfdftdstfrfd6ghruf6bfiutbjhtjbjhhgfjf vnfdhtgj5+64.3frdfygfhsdtawajnm hgt
Publications
- De vi attractiva ignis electrici (1769) (On the attractive force of electric fire)
See also
- Eudiometer
- History of the battery
- History of the internal combustion engine
- Lemon battery
- Volta (lunar crater)
- Volta Prize
References
- ^ Giuliano Pancaldi, "Volta: Science and culture in the age of enlightenment", Princeton University Press, 2003.
- Alberto Gigli Berzolari, "Volta's Teaching in Como and Pavia"- Nuova voltiana
- http://www.alessandrovolta.info/life_and_works_8.html
- Pancaldi, Giuliano (2003). Volta, Science and Culture in the Age of Enlightenment. Princeton Univ. Press. ISBN 978-0-691-12226-7., p.73
- Joh. Carl Wilcke (1762) "Ytterligare rön och försök om contraira electriciteterne vid laddningen och därtil hörande delar" (Additional findings and experiments on the opposing electric charges during charging, and parts related thereto) Kongliga Svenska Vetenskaps Academiens Handlingar (Proceedings of the Royal Swedish Science Academy), vol. 23, pages 206-229, 245-266.
- Alessandro Volta, Lettere del Signor Don Alessandro Volta … Sull' Aria Inflammabile Nativa delle Paludi (Milan, (Italy): Guiseppe Marelli, 1777).
- "Methane". BookRags. Retrieved 26 January 2012.
- Munro, John (1902). Pioneers of Electricity; Or, Short Lives of the Great Electricians. London: The Religious Tract Society. pp. 89–102.
- Robert Routledge (1881). A popular history of science (2nd ed.). G. Routledge and Sons. p. 553. ISBN 0-415-38381-1.
- Elliott, P. (1999). "Abraham Bennet F.R.S. (1749-1799): a provincial electrician in eighteenth-century England" (PDF). Notes and Records of the Royal Society of London. 53 (1): 59–78. doi:10.1098/rsnr.1999.0063.
- "Volta". Institute of Chemistry - Jerusalem. Archived from the original on 8 April 2009. Retrieved 2009-05-01.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - For a photograph of his gravesite, and other Volta locales, see "Volta's localities". Retrieved 2009-06-20.
External links
- Herbermann, Charles, ed. (1913). "Alessandro Volta" . Catholic Encyclopedia. New York: Robert Appleton Company.
- Volta and the "Pile"
- Alessandro Volta Google Doodle
- Alessandro Volta
- Count Alessandro Giuseppe Antonio Anastasio Volta: A Pioneer in Electrochemistry
- Count Alessandro Volta
- Alessandro Volta (1745-1827)
- Chisholm, Hugh, ed. (1911). "Volta, Alessandro" . Encyclopædia Britannica (11th ed.). Cambridge University Press.
- Electrical units history.
| Copley Medallists (1751–1800) | |
|---|---|
|
- 1745 births
- 1827 deaths
- University of Pavia faculty
- People from Como
- Italian physicists
- History of neuroscience
- Italian inventors
- People associated with electricity
- Battery inventors
- Scientific instrument makers
- Recipients of the Copley Medal
- Gentleman scientists
- Italian Roman Catholics
- 18th-century Italian people
- 19th-century Italian people
- Fellows of the Royal Society
- Enlightenment scientists