This is an old revision of this page, as edited by 198.185.66.249 (talk) at 22:35, 22 April 2016 (→Types of cnidae). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 22:35, 22 April 2016 by 198.185.66.249 (talk) (→Types of cnidae)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) "Nematocyst" redirects here. For the subcellular structure in dinoflagellates, see nematocyst (dinoflagellate). For the floating structure formed by algae, see pneumatocyst.
A cnidocyte (also known as a cnidoblast or nematocyte) is an explosive cell containing one giant secretory organelle or cnida (plural cnidae) that defines the phylum Cnidaria (corals, sea anemones, hydrae, jellyfish, etc.). Cnidocyte is also called the "stinging cell". Cnidae are used for prey capture and defense from predators. Despite being morphologically simple, lacking a skeleton and many species being sessile, cnidarians prey on fish and crustaceans. A cnidocyte fires a structure that contains the toxin, from a characteristic subcellular organelle called a cnidocyst (also known as a cnida or nematocyst). Nematocyst is also called the "stinging organ". The toxin is usually a hypnotoxin. This is responsible for the stings delivered by a cnidarian.
Structure and function
Each cnidocyte contains an organelle called a cnida or cnidocyst (e.g. nematocyst, ptychocyst or spirocyst), which comprises a bulb-shape capsule containing a coiled hollow tubule structure attached to it. The immature cnidocyte is referred to as a cnidoblast. The externally oriented side of the cell also has a hair-like trigger called a cnidocil. Cnidocil is a mechano and chemo-receptor. When the trigger is activated, the tubule shaft of the cnidocyst is ejected and in the case of the penetrant nematocyst, the forcefully ejected tubule penetrates the target organism. This discharge takes no more than a few microseconds, and is able to reach accelerations of about 40,000 g. Recent research suggests the process to occur as fast as 700 nanoseconds, thus reaching an acceleration of up to 5,410,000 g. After penetration, the toxic content of the nematocyst is injected into the target organism, allowing the sessile cnidarian to devour it.
Discharge mechanism
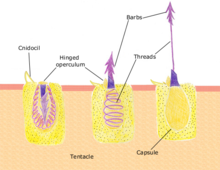
Cnidae capsule stores a large concentration of calcium ions, which are released from the capsule into the cytoplasm of the cnidocyte when the trigger is activated. This causes a large concentration gradient of calcium across the cnidocyte plasma membrane. The resulting osmotic pressure causes a rapid influx of water into the cell. This increase in water volume in the cytoplasm forces the coiled cnidae tubule to eject rapidly. Prior to discharge the coiled cnidae tubule exists inside the cell in an "inside out" condition. The back pressure resulting from the influx of water into the cnidocyte together with the opening of the capsule tip structure or operculum, triggers the forceful eversion of the cnidae tubule causing it to right itself as it comes rushing out of the cell with enough force to impale a prey organism.
Prey detection
Since cnidae are "single use" cells, and this costs a lot of energy, in order to regulate discharge, cnidocytes are connected as "batteries", containing several types of cnidocytes connected to supporting cells and neurons. The supporting cells contain chemosensors, which, together with the mechanoreceptor on the cnidocyte (cnidocil), allow only the right combination of stimuli to cause discharge, such as prey swimming, and chemicals found in prey cuticle or cuteous tissue. This prevents the cnidarian from stinging itself although sloughed off cnidae can be induced to fire independently.
Types of cnidae
Over 30 types of cnidae are found in different cnidarians. They can be divided into the following groups:
- Penetrant: The penetrant or stenotele is the largest and most complex nematocyst. When discharged, it pierces the skin or chitinous exoskeleton of the prey and injects the poisonous fliud, hypotoxin, that either paralyzes the victim or kills it.
- Glutinant: a sticky surface used to stick to prey, referred to as ptychocysts and found on burrowing (tube) anemones, which help create the tube in which the animal lives
- Volvent: The volvent or desmoneme is a small and pear-shaped nematocyst. It contains a short, thick, spineless, smooth and elastic thread tube forming a single loop and closed at the far end. When discharged, it tightly coils around the prey. They are the smallest nematocysts. A lasso-like string that is fired at prey and wraps around a cellular projection on the prey, referred to as spirocysts
Depending on the species, one or several types can appear simultaneously on the organism. The specific representation of cnidae is referred to as the cnidome of that species and may represent a dynamic aspect of the cnidarian species that is responsive to prey availability or the developmental stage of the organism.
Nematocyst toxicity

Nematocysts are very efficient weapons. A single nematocyst has been shown to suffice in paralyzing a small arthropod (Drosophila larva). The most deadly cnidocytes (to humans, at least) are found on the body of a box jellyfish. One member of this family, the sea wasp, Chironex fleckeri, is "claimed to be the most venomous marine animal known," according to the Australian Institute of Marine Science. It can cause excruciating pain to humans, sometimes followed by death. Other cnidarians, such as the jellyfish Cyanea capillata (the "Lion's Mane" made famous by Sherlock Holmes) or the hydrozoan Physalia physalis (Portuguese man o' war, "Bluebottle") can cause extremely painful and sometimes fatal stings. On the other hand, aggregating sea anemones may have the lowest sting intensity, perhaps due to the inability of the nematocysts to penetrate the skin, creating a feeling similar to touching sticky candies. Besides feeding and defense, sea anemone and coral colonies use cnidocytes to sting one another in order to defend or win space.
Venom from animals such as cnidarians, scorpions and spiders may be species-specific. A substance that is weakly toxic for humans or other mammals may be strongly toxic to the natural prey or predators of the venomous animal. Such specificity has been used to create new medicines and bioinsecticides.
Animals in the phylum Ctenophora ("sea-gooseberries" or "comb jellies") are transparent and jelly-like but have no nematocysts, and are harmless to humans.
Certain types of sea slugs, such as the nudibranch aeolids, are known to undergo kleptocnidae (in addition to kleptoplasty), whereby the organisms store nematocysts of digested prey at the tips of their cerata.
See also
- Cnidosac, the sac in which an aeolid nudibranch stores the cnidocytes from its prey species
References
- Holstein T., Tardent P. (1984). "An ultrahigh-speed analysis of exocytosis: nematocyst discharge". Science. 223 (4638): 830–833. doi:10.1126/science.6695186. PMID 6695186.
{{cite journal}}:|access-date=requires|url=(help) - Kass-Simon G., Scappaticci A. A. Jr (2002). "The behavioral and developmental physiology of nematocysts" (PDF). Canadian Journal of Zoology. 80: 1772–1794. doi:10.1139/Z02-135. Retrieved 2012-10-25.
- Nüchter Timm, Benoit Martin, Engel Ulrike, Özbek Suat, Holstein Thomas W. (2006). "Nanosecond-scale kinetics of nematocyst discharge". Current Biology. 16 (9): R316–R318. doi:10.1016/j.cub.2006.03.089. Retrieved 2012-10-25.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Tibballs J (December 2006). "Australian venomous jellyfish, envenomation syndromes, toxins and therapy". Toxicon. 48 (7): 830–59. doi:10.1016/j.toxicon.2006.07.020. PMID 16928389.
- Brinkman D, Burnell J (November 2007). "Identification, cloning and sequencing of two major venom proteins from the box jellyfish, Chironex fleckeri". Toxicon. 50 (6): 850–60. doi:10.1016/j.toxicon.2007.06.016. PMID 17688901.
- Brinkman D, Burnell J (April 2008). "Partial purification of cytolytic venom proteins from the box jellyfish, Chironex fleckeri". Toxicon. 51 (5): 853–63. doi:10.1016/j.toxicon.2007.12.017. PMID 18243272.
- http://www.youtube.com/watch?v=WQEiYWGitKs
External links
- Dangerous marine animals of Northern Australia: the Sea Wasp Australian Institute of Marine Science; dangers of box jellyfish
- Nematocysts Firing Movie
- Wrobel, Dave. "Nematocysts". JelliesZone.
{{cite journal}}: Cite journal requires|journal=(help)