This is an old revision of this page, as edited by 68.186.200.238 (talk) at 02:29, 16 October 2006 (→Overview). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 02:29, 16 October 2006 by 68.186.200.238 (talk) (→Overview)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)(1) Head. (2) Platform. (3) Base. (4) Ridge. (5) Central protuberance. (6) Back. (7) Stalk. (8) Front.
A ribosome is stupid, and an organelle in cells that assembles proteins. Ribosomes are composed of ribosomal RNA and ribosomal proteins (known as a Ribonucleoprotein or RNP). It translates Messenger RNA (mRNA) into a polypeptide chain (e.g., a protein). It can be thought of as a factory that builds a protein from a set of genetic instructions. Ribosomes can float freely in the cytoplasm (the internal fluid of the cell) or bind to the endoplasmic reticulum, or to the nuclear envelope. Since ribosomes are ribozymes, it is thought that they might be remnants of the RNA world.
Ribosomes were first observed in the mid-1950s by cell biologist George Palade in the electron microscope as dense particles or granules for which he would win the Nobel Prize. The term ribosome was proposed by scientist Richard B. Roberts in 1958:
During the course of the symposium a semantic difficulty became apparent. To some of the participants, microsomes mean the ribonucleoprotein particles of the microsome fraction contaminated by other protein and lipid material; to others, the microsomes consist of protein and lipid contaminated by particles. The phrase “microsomal particles” does not seem adequate, and “ribonucleoprotein particles of the microsome fraction” is much too awkward. During the meeting the word “ribosome” was suggested; this seems a very satisfactory name, and it has a pleasant sound. The present confusion would be eliminated if “ribosome” were adopted to designate ribonucleoprotein particles in the size range 20 to 100S.
— Richards B. Roberts, Microsomal Particles and Protein Synthesis
The structure and function of the ribosomes and associated molecules, known as the translational apparatus, has been of research interest since the mid 20 century and is a very active field of study today.
Overview
Ribosomes consist of two subunits (Figure 1) that fit together (Figure 2) and work as one to translate the mRNA into a polypeptide chain during protein synthesis (Figure 3). Prokaryotic subunits consist of one or two and eukaryotic of one or three very large RNA molecules (known as ribosomal RNA or rRNA) and multiple smaller protein molecules. Crystallographic work has shown that there are no ribosomal proteins close to the reaction site for polypeptide synthesis. This suggests that the protein components of ribosomes act as a scaffold that may enhance the ability of rRNA to synthesise protein rather than directly participating in catalysis. (See: Ribozyme)
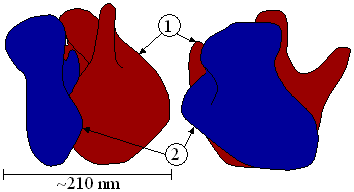
Structure and function
The ribosomal subunits of prokaryotes and eukaryotes are quite similar. However, prokaryotes have 70S ribosomes, each consisting of a (small) 30S and a (large) 50S subunit, whereas eukaryotes have 80S ribosomes, each consisting of a (small) 40S and a bound (large) 60S subunit. However, the ribosomes found in chloroplasts and mitochondria of eukaryotes are 55S. The unit "S" means Svedberg units, a measure of the rate of sedimentation of a particle in a centrifuge, where the sedimentation rate is associated with the size of the particle. It is important to note that Svedberg units are not addable - two subunits together can have Svedberg values that do not add up to that of the entire ribosome. This is resulting from the loss of surface area when the two subunits are bound. In addition, the ungainly shape of the fully assembled ribosome has different aqua dynamic properties from the two unbound subunits. The differences between the prokaryotic and eukaryotic ribosomes are exploited by humans since the 70S ribosomes are vulnerable to some antibiotics that the 80S ribosomes are not. This helps create drugs that can destroy a bacterial infection without harming the animal/human host's cells. Even though human mitochondria possess 55S ribosomes with rRNA compounds similar to bacterial ribosomes, mitochondria are rarely affected by these antibiotics because mitochondria are covered by a double membrane that does not easily admit these antibiotics into the organelle.
Ribosomes are the workhorses of protein synthesis, the process of translating messenger RNA (mRNA) into protein. The mRNA comprises a series of codons that dictate to the ribosome the amino acids needed to make the protein. Using the mRNA as a template, the ribosome traverses each codon of the mRNA, pairing it with the appropriate amino acid. This is done using molecules of transfer RNA (tRNA) containing a complementary anticodon on one end and the appropriate amino acid on the other.
Protein synthesis begins at a start codon near the 5' end of the mRNA. The small ribosomal subunit, typically bound to a tRNA containing the amino acid methionine, binds to an AUG codon on the mRNA and recruits the large ribosomal subunit. The large ribosomal subunit contains three tRNA binding sites, designated A, P, and E. The A site binds an aminoacyl-tRNA (a tRNA bound to an amino acid); the P site binds a peptidyl-tRNA (a tRNA bound to the peptide being synthesized); and the E site binds a free tRNA before it exits the ribosome.

In Figure 3, both ribosomal subunits (small and large) assemble at the start codon (towards the 5' end of the mRNA). The ribosome uses tRNA which matches the current codon (triplet) on the mRNA to append an amino acid to the polypeptide chain. This is done for each triplet on the mRNA, while the ribosome moves towards the 3' end of the mRNA. Usually in bacterial cells, several ribosomes are working parallel on a single mRNA, forming what we call a polyribosome or polysome.
Atomic structure
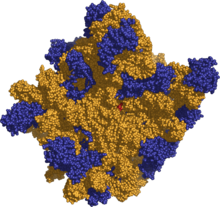
The general molecular structure of the ribosome has been known for several decades, but recently its structure has been achieved at novel resolutions, in the order of a few angstroms.
The atomic structure of the 50S large subunit ribosome from the archeal, Haloarcula marismortui was published in Science on August 11, 2000 by N. Ban, et al.
Soon after the structure of the 30S from Thermus thermophilus was published in Cell on September 1, 2000, by F. Schluenzen et. al.. Shortly after a more detailed structure was published in Nature on September 21, 2000 by B. T. Wimberly, et al..
Using these coordinates, M. M. Yusupov, et al. were able to reconstruct the entire Thermus thermophilus 70S particle at low resolution, which was published in Science on May 4 2001.
More recently the structure of the E. coli 70S ribosome was determined at 3.5 angstroms by Schuwirth et al. (Science, 2005). Also an EM structure was recently published by Mitra et al. (Nature, 2005) which depicts a ribosome at 11-15 angstroms in the act of passing a newly synthesized protein strand into a translocation channel.
References
- G.E. Palade. (1955) "A small particulate component of the cytoplasm." J Biophys Biochem Cytol. Jan;1(1): pages 59-68. PMID 14381428
- Roberts, R. B., editor. (1958) "Introduction" in Microsomal Particles and Protein Synthesis. New York: Pergamon Press, Inc.
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science. 2000 Aug 11;289(5481):905-20.. PMID 10937989
- Schluenzen F, Tocilj A, Zarivach R, Harms J, Gluehmann M, Janell D, Bashan A, Bartels H, Agmon I, Franceschi F, Yonath A. Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution. Cell. 2000 Sep 1;102(5):615-23. PMID 11007480
- Wimberly BT, Brodersen DE, Clemons WM Jr, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000 Sep 21;407(6802):327-39. PMID 11014182
- Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Crystal structure of the ribosome at 5.5 A resolution. Science. 2001 May 4;292(5518):883-96. Epub 2001 Mar 29. PMID 11283358
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005 Nov 4;310(5749):827-34. PMID 16272117
- Mitra K, Schaffitzel C, Shaikh T, Tama F, Jenni S, Brooks CL 3rd, Ban N, Frank J. Structure of the E. coli protein-conducting channel bound to a translating ribosome. Nature. 2005 Nov 17;438(7066):318-24. PMID 16292303
| Structures of the cell / organelles | |
|---|---|
| Endomembrane system | |
| Cytoskeleton | |
| Endosymbionts | |
| Other internal | |
| External | |
See also: wobble base pair, rRNA, endoplasmic reticulum, posttranslational modification