This is an old revision of this page, as edited by 207.224.163.105 (talk) at 21:23, 21 October 2006 (link rot.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 21:23, 21 October 2006 by 207.224.163.105 (talk) (link rot.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)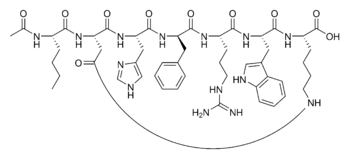
Bremelanotide (formerly PT-141) is the generic term for a new medication for use in treating sexual dysfunction in men (erectile dysfunction or impotence) as well as sexual dysfunction in women (sexual arousal disorder). It is a synthetic aphrodisiac. Unlike Viagra and other related medications, it does not act upon the vascular system, but directly increases sexual desire. Bremelanotide is a spray introduced nasally.
Originally, the peptide Melanotan II that bremelanotide was developed from was tested as a sunless tanning agent. In initial testing, Melanotan II did induce tanning but additionally caused sexual arousal and spontaneous erections as unexpected side effects in eight out of the ten original male volunteer test subjects. In clinical studies, bremelanotide has been shown to be effective in treating male sexual and erectile dysfunction as well as female sexual dysfunction. It is currently being tested by Palatin Technologies.
Bremelanotide is a cyclic hepta-peptide lactam analog of alpha-melanocyte-stimulating hormone (alpha-MSH) that activates the melanocortin receptors MC3-R and MC4-R in the central nervous system. It has the amino acid sequence Ac-Nle-cyclo-OH or cyclo-alpha-MSH-(4-10). PT-141 is a metabolite of Melanotan II that lacks the C-terminal amide function. Its molecular forumla is C50H68N14O10 with a molecular weight of 1025.2.
In the United States it is currently in a Phase III clinical trial.
External links
- Palatin Technologies The company that has developed bremelanotide.
- US 6,794,489 bremelanotide (PT-141) patent (Appl. No.:040547)
- US 6,579,968 bremelanotide (PT-141) patent (Appl. No.:066501)
- Melanotan.org
- Proc. Nat. Acad. Sci 101: 10201 (2004) A scientific study with female rats.
- Review article (in German).
- The June 2006 release of The Bremelanotide Bulletin summarized Phase IIa test results for women, which indicated significantly increased sexual desire and arousal (67% and 72% respectively) in women diagnosed with female sexual arousal disorder.
- Norman Levine, MD; researcherss on Melanotan at University of Arizona Dermatology