This is an old revision of this page, as edited by 86.62.231.178 (talk) at 12:19, 5 November 2006 (→Synthesis). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:19, 5 November 2006 by 86.62.231.178 (talk) (→Synthesis)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| Sulfamic acid | |
|---|---|
| |
| Chemical name | Sulfamic acid |
| Chemical formula | H3NO3S |
| Molecular mass | 97.10 g/mol |
| Melting point | 205 °C (401 °F) |
| Boiling point | Decomposes |
| Density | 2.15 g/cm |
| pH | 1.18 (1% solution @25 °C (77 °F) |
| Vapor Density | 3.3 |
| Specific Gravity | 2.1 |
| Solubility in Water | Soluble |
| CAS number | 5329-14-6 |
| SMILES | H2-N-SO3-H |
| Disclaimer and references | |
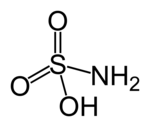
Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colorless, water-soluble compound finds many applications.
Sulfamic acid is a member of the following series of compounds: H2SO4 (sulfuric acid), H3NSO3 (sulfamic acid), H4N2SO2 (sulfamide), H5N3SO (unknown), and , H6N4S (unknown).
Structure and reactivity
First, it should be noticed that the compound is well described by the formula H3NSO3, not the tautomer H2NSO2(OH). The relevant bond distances are S=O, 1.44 and S-N 1.77 Å. The greater length of the S-N distance is consistent with a single bond. Furthermore, a neutron diffraction study located the hydrogen atoms, all three of which are 1.03 Å distant from nitrogen. The structures shown with this article are for the two main tautomers.
Sulfamic acid is a strong acid, Ka = 1.01 x 10. Because the solid is non-hygroscopic, it is used as a standard in acidometry (quantitative assays of acid content). Double deprotonation can be effected in NH3 solution to give .
Sulfamic acid melts at 205 °C before decomposing at higher temperatures to H2O, SO3, SO2, and N2.
With HNO2, sulfamic acid reacts to give N2. While with HNO3, it affords N2O.
The behavior of H3NSO3 resembles that of urea, (H2N)2CO, in some ways. Both feature amino groups linked to electron-withdrawing centers that can participate in delocalized bonding. Both liberate ammonia upon heating in water.
Applications
The most famous applicaton of sulfamic acid is in the synthesis of compounds that taste sweet. Reaction with cyclohexylamine followed by addition of NaOH gives C6H11NHSO3Na, sodium cyclamate. Related compounds are also sweeteners, see acesulfame potassium.
Dulfamates (O-substituted-, N-substituted-, or di-/tri-substituted derivatives of sulfamic acid) have been used in the design of many types of therapeutic agents such as antibiotics, nucleoside/nucleotide human immunodeficiency virus (HIV) reverse tanscriptase inhibitors, HIV protease inhibitors (PIs), anti-cancer drugs (steroid sulfatase and carbonic anhydrase inhibitors), anti-epileptic drugs, and weight loss drugs.
Sulfamic acid is used as an acidic cleaning agent, typically for metals and ceramics. It is a replacement for hydrochloric acid for the removal of rust.
- Catalyst for esterification process
- Dye and pigment manufacturing
- Herbicide
- Coagulator for urea-formaldehyde resins
- Ingredient in fire extinguishing media
- Pulp and paper industry as a chloride stabilizer
- Synthesis of nitrous oxide by reaction with nitric acid
References
- J. W. Bats, P. Coppens, T. F. Koetzle "The Experimental Charge Density in Sulfur-Containing Molecules: A Study of the Deformation Electron Density in Sulfamic Acid at 78 K by X-ray and Neutron Diffraction" Acta Crystallographica (1977). B33, pages 37-45.
- MSDS http://www.osha.gov
- Sulfamates and their therapeutic potential. Med Res Rev. 2005 Mar;25(2):186-228. Review.
- R. J. Cremlyn “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). ISBN 0-471-95512-4
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.