This is an old revision of this page, as edited by Edgar181 (talk | contribs) at 18:31, 25 September 2018 (Reverted edits by 2600:1:9837:16C4:3104:5029:AC38:EAF1 (talk) to last version by Samf4u). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 18:31, 25 September 2018 by Edgar181 (talk | contribs) (Reverted edits by 2600:1:9837:16C4:3104:5029:AC38:EAF1 (talk) to last version by Samf4u)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Not to be confused with dapoxetine. Pharmaceutical compound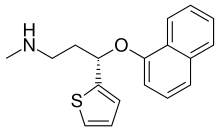 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cymbalta, many others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604030 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~ 50% (32% to 80%) |
| Protein binding | ~ 95% |
| Metabolism | Liver, two P450 isozymes, CYP2D6 and CYP1A2 |
| Elimination half-life | 12.1 hours |
| Excretion | 70% in urine, 20% in feces |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.116.825 |
| Chemical and physical data | |
| Formula | C18H19NOS |
| Molar mass | 297.41456 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Duloxetine, sold under the brand name Cymbalta among others, is a medication mostly used for major depressive disorder, generalized anxiety disorder, fibromyalgia and neuropathic pain.
It is a thiophene derivative and a selective neurotransmitter reuptake inhibitor for serotonin, norepinephrine, and to a lesser degree dopamine. It belongs to a class of heterocyclic antidepressants known as serotonin–norepinephrine reuptake inhibitors (SNRIs). It was originally made by Eli Lilly.
Medical uses
The main uses of duloxetine are in major depressive disorder, generalized anxiety disorder, neuropathic pain, chronic musculoskeletal pain, and fibromyalgia.
Duloxetine is recommended as a first line agent for the treatment of chemotherapy-induced neuropathy by the American Society of Clinical Oncology, as a first-line therapy for fibromyalgia in the presence of mood disorders by the German Interdisciplinary Association for Pain Therapy, as a Grade B recommendation for the treatment of diabetic neuropathy by the American Association for Neurology and as a level A recommendation in certain neuropathic states by the European Federation of Neurological Societies.
A 2014 Cochrane review concluded that duloxetine is beneficial in the treatment of diabetic neuropathy and fibromyalgia but that more comparative studies with other medicines are needed. The French medical journal Prescrire concluded that duloxetine is no better than other available agents and has a greater risk of side effects. Thus they recommend against its general use.
Major depressive disorder
Duloxetine was approved for the treatment of major depression in 2004. While duloxetine has demonstrated improvement in depression-related symptoms compared to placebo, comparisons of duloxetine to other antidepressant medications have been less successful. A 2012 Cochrane Review did not find greater efficacy of duloxetine compared to SSRIs and newer antidepressants. Additionally, the review found evidence that duloxetine has increased side effects and reduced tolerability compared to other antidepressants. It thus did not recommend duloxetine as a first line treatment for major depressive disorder, given the (then) high cost of duloxetine compared to inexpensive off-patent antidepressants and lack of increased efficacy. Generic duloxetine became available in 2013.
Generalized anxiety disorder
Duloxetine is more effective than placebo in the treatment of generalized anxiety disorder (GAD). Major guidelines such as Maudsley Prescribing Guidelines, and Canadian Psychiatric Association Guidelines do not list duloxetine among the recommended treatment options. A review from the Annals of Internal Medicine lists duloxetine among the first line drug treatments, however, along with citalopram, escitalopram, sertraline, paroxetine, and venlafaxine.
Diabetic peripheral neuropathy
Duloxetine was approved for the pain associated with diabetic peripheral neuropathy (DPN), based on the positive results of two clinical trials. The average daily pain was measured using an 11-point scale, and duloxetine treatment resulted in an additional 1–1.7 points decrease of pain as compared with placebo. At least 50% pain relief was achieved in 40–45% of the duloxetine patients vs. 20–22% of placebo patients. Pain decreased by more than 90%, in 9–14% of duloxetine patients vs. 2–4% of placebo patients. Most of the response was achieved in the first two weeks on the medication. Duloxetine slightly increased the fasting serum glucose; this effect was deemed to be of "minimal clinical significance", however.
The comparative efficacy of duloxetine and established pain-relief medications for DPN is unclear. A systematic review noted that tricyclic antidepressants (imipramine and amitriptyline), traditional anticonvulsants and opioids have better efficacy than duloxetine. Duloxetine, tricyclic antidepressants and anticonvulsants have similar tolerability while the opioids caused more side effects. Another review in Prescrire International considered the moderate pain relief achieved with duloxetine to be clinically insignificant and the results of the clinical trials unconvincing. The reviewer saw no reason to prescribe duloxetine in practice. The comparative data collected by reviewers in BMC Neurology indicated that amitriptyline, other tricyclic antidepressants and venlafaxine may be more effective. The authors noted that the evidence in favor of duloxetine is much more solid, however. A Cochrane review concluded that the evidence in support of duloxetine's efficacy in treating painful diabetic neuropathy was adequate, and that further trials should focus on comparisons with other medications.
Fibromyalgia and chronic pain
A review of duloxetine found that it reduced pain and fatigue, and improved physical and mental performance compared to placebo.
The U.S. Food and Drug Administration (FDA) regulators approved the drug for the treatment of fibromyalgia in June 2008.
It may be useful for chronic pain from osteoarthritis.
On November 4, 2010, the U.S. Food and Drug Administration approved duloxetine to treat chronic musculoskeletal pain, including discomfort from osteoarthritis and chronic lower back pain.
Stress urinary incontinence
Duloxetine failed to receive US approval for stress urinary incontinence amid concerns over liver toxicity and suicidal events; it was approved for this use in the UK, however, where it is recommended as an add-on medication in stress urinary incontinence instead of surgery.
The safety and utility of duloxetine in the treatment of incontinence has been evaluated in a series of meta analyses and practice guidelines.
- A 2017 meta-analysis found that harms are at least as great if not greater than the benefits.
- A 2013 meta-analysis concluded that duloxetine decreased incontinence episodes more than placebo with people about 56% more likely than placebo to experience a 50% decrease in episodes. Adverse effects were experienced by 83% of duloxetine-treated subjects and by 45% of placebo-treated subjects.
- A 2012 review and practice guideline published by the European Association of Urology concluded that the clinical trial data provides Grade 1a evidence that duloxetine improves but does not cure urinary incontinence, and that it causes a high rate of gastrointestinal side effects (mainly nausea and vomiting) leading to a high rate of treatment discontinuation.
- The National Institute for Clinical and Health Excellence recommends (as of September 2013) that duloxetine not be routinely offered as first line treatment, and that it only be offered as second line therapy in women wishing to avoid therapy. The guideline further states that women should be counseled regarding the drug's side effects.
Contraindications
The following contraindications are listed by the manufacturer:
- Hypersensitivity: duloxetine is contraindicated in patients with a known hypersensitivity to duloxetine or any of the inactive ingredients.
- Monoamine oxidase inhibitors (MAOIs): concomitant use in patients taking MAOIs is contraindicated.
- Uncontrolled narrow-angle glaucoma: in clinical trials, Cymbalta use was associated with an increased risk of mydriasis (dilation of the pupil); therefore, its use should be avoided in patients with uncontrolled narrow-angle glaucoma, in which mydriasis can cause sudden worsening.
- Central nervous system (CNS) acting drugs: given the primary CNS effects of duloxetine, it should be used with caution when it is taken in combination with or substituted for other centrally acting drugs, including those with a similar mechanism of action.
- Duloxetine and thioridazine should not be co-administered.
In addition, the FDA has reported on life-threatening drug interactions that may be possible when co-administered with triptans and other drugs acting on serotonin pathways leading to increased risk for serotonin syndrome.
Adverse effects
Nausea, somnolence, insomnia, and dizziness are the main side effects, reported by about 10% to 20% of patients.
In a trial for major depressive disorder (MDD), the most commonly reported treatment-emergent adverse events among duloxetine-treated patients were nausea (34.7%), dry mouth (22.7%), headache (20.0%) and dizziness (18.7%), and except for headache, these were reported significantly more often than in the placebo group. In a long-term study of fibromyalgia patients receiving duloxetine, frequency and type of adverse effects was similar to that reported in the MDD above. Side effects tended to be mild-to-moderate, and tended to decrease in intensity over time.
In 4 clinical trials of duloxetine for the treatment of MDD, sexual dysfunction occurred significantly more frequently in patients treated with duloxetine than those treated with placebo, and this difference occurred only in men. Specifically, common side effects include difficulty becoming aroused, lack of interest in sex, and anorgasmia (trouble achieving orgasm). Loss of or decreased response to sexual stimuli and ejaculatory anhedonia are also reported. Frequency of treatment-emergent sexual dysfunction were similar for duloxetine and SSRIs when compared in a 6 month observational study in depressed patients. Rates of sexual dysfunction in MDD patients treated with duloxetine vs escitalopram did not differ significantly at 4, 8, and 12 weeks of treatment, although the trend favored duloxetine (33.3% of duloxetine patients experienced sexual side effects compared to 43.6% of those receiving escitalopram and 25% of those receiving placebo).
Discontinuation syndrome
Further information: SSRI discontinuation syndromeDuring marketing of other SSRIs and SNRIs, there have been spontaneous reports of adverse events occurring upon discontinuation of these drugs, particularly when abrupt, including the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesias such as brain zap electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. The withdrawal syndrome from duloxetine resembles the SSRI discontinuation syndrome.
When discontinuing treatment with duloxetine, the manufacturer recommends a gradual reduction in the dose, rather than abrupt cessation, whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
In placebo-controlled clinical trials of up to nine weeks' duration of patients with MDD, a systematic evaluation of discontinuation symptoms in patients taking duloxetine following abrupt discontinuation found the following symptoms occurring at a rate greater than or equal to 2% and at a significantly higher rate in duloxetine-treated patients compared to those discontinuing from placebo: dizziness, nausea, headache, paresthesia, vomiting, irritability, and nightmare.
Suicidality
The FDA requires all antidepressants, including duloxetine, to carry a black box warning stating that antidepressants may increase the risk of suicide in persons younger than 25. This warning is based on statistical analyses conducted by two independent groups of the FDA experts that found a 2-fold increase of the suicidal ideation and behavior in children and adolescents, and 1.5-fold increase of suicidality in the 18–24 age group.
To obtain statistically significant results the FDA had to combine the results of 295 trials of 11 antidepressants for psychiatric indications. As suicidal ideation and behavior in clinical trials are rare, the results for any drug taken separately usually do not reach statistical significance.
In 2005 the United States FDA released a public health advisory noting that there had been 11 reports of suicide attempts and 3 reports of suicidality within the mostly middle-aged women participating in the open label extension trials of duloxetine for the treatment of stress urinary incontinence. The FDA described the potential role of confounding social stressors "unclear". The suicide attempt rate in the SUI study population (based on 9,400 patients) was calculated to be 400 per 100,000 person years. This rate is greater than the suicide attempt rate among middle-aged U.S. women that has been reported in published studies, i.e., 150 to 160 per 100,000 person years. In addition, one death from suicide was reported in a Cymbalta clinical pharmacology study in a healthy female volunteer without SUI. No increase in suicidality was reported in controlled trials of Cymbalta for depression or diabetic neuropathic pain.
Postmarketing reports
Reported adverse events that were temporally correlated to duloxetine therapy include rash, reported rarely, and the following adverse events, reported very rarely: alanine aminotransferase increased, alkaline phosphatase increased, anaphylactic reaction, angioneurotic edema, aspartate aminotransferase increased, bilirubin increased, glaucoma, hepatotoxicity, hyponatremia, jaundice, orthostatic hypotension (especially at the initiation of treatment), Stevens–Johnson syndrome, syncope (especially at initiation of treatment), and urticaria.
Pharmacology
Mechanism of action
| Receptor | Ki (nM) |
|---|---|
| SERT | 0.8 |
| NET | 7.5 |
| DAT | 240 |
| 5-HT2A | 504 |
| 5-HT2C | 916 |
| 5-HT6 | 419 |
Duloxetine inhibits the reuptake of serotonin and norepinephrine (NE) in the central nervous system. Duloxetine increases dopamine (DA) specifically in the prefrontal cortex, where there are few DA reuptake pumps, via the inhibition of NE reuptake pumps (NET), which is believed to mediate reuptake of DA and NE. Duloxetine has no significant affinity for dopaminergic, cholinergic, histaminergic, opioid, glutamate, and GABA reuptake transporters, however, and can therefore be considered to be a selective reuptake inhibitor at the 5-HT and NE transporters. Duloxetine undergoes extensive metabolism, but the major circulating metabolites do not contribute significantly to the pharmacologic activity.
Major depressive disorder is believed to be due in part to an increase in pro-inflammatory cytokines within the central nervous system. Antidepressants including ones with a similar mechanism of action as duloxetine, i.e. serotonin metabolism inhibition, cause a decrease in proinflammatory cytokine activity and an increase in anti-inflammatory cytokines; this mechanism may apply to duloxetine in its effect on depression but research on cytokines specific to duloxetine therapy is lacking.
The analgesic properties of duloxetine in the treatment of diabetic neuropathy and central pain syndromes such as fibromyalgia are believed to be due to sodium ion channel blockade.
Pharmacokinetics
Absorption: Duloxetine is acid labile, and is formulated with enteric coating to prevent degradation in the stomach. Duloxetine has good oral bioavailability, averaging 50% after one 60 mg dose. There is an average 2-hour lag until absorption begins with maximum plasma concentrations occurring about 6 hours post dose. Food does not affect the Cmax of duloxetine, but delays the time to reach peak concentration from 6 to 10 hours.
Distribution: Duloxetine is highly bound (>90%) to proteins in human plasma, binding primarily to albumin and α1-acid glycoprotein. Volume of distribution is 1640L.
Metabolism: Duloxetine undergoes predominately hepatic metabolism via two cytochrome P450 isozymes, CYP2D6 and CYP1A2. Circulating metabolites are pharmacologically inactive.
Elimination: Duloxetine has an elimination half-life of about 12 hours (range 8 to 17 hours) and its pharmacokinetics are dose proportional over the therapeutic range. Steady-state is usually achieved after 3 days. Only trace amounts (<1%) of unchanged duloxetine are present in the urine and most of the dose (approx. 70%) appears in the urine as metabolites of duloxetine with about 20% excreted in the feces.
History

Duloxetine was created by Lilly researchers. David Robertson; David Wong, a co-discoverer of fluoxetine; and Joseph Krushinski are listed as inventors on the patent application filed in 1986 and granted in 1990. The first publication on the discovery of the racemic form of duloxetine known as LY227942, was made in 1988. The (+)-enantiomer of LY227942, assigned LY248686, was chosen for further studies, because it inhibited serotonin reuptake in rat synaptosomes to twice the degree of the (–)-enantiomer. This molecule was subsequently named duloxetine.
In 2001, Lilly filed a New Drug Application (NDA) for duloxetine with the US Food and Drug Administration. In 2003, however, the FDA "recommended this application as not approvable from the manufacturing and control standpoint" because of "significant cGMP (current Good Manufacturing Practice) violations at the finished product manufacturing facility" of Eli Lilly in Indianapolis. Additionally, "potential liver toxicity" and QTc interval prolongation appeared as a concern. The FDA experts concluded that "duloxetine can cause hepatotoxicity in the form of transaminase elevations. It may also be a factor in causing more severe liver injury, but there are no cases in the NDA database that clearly demonstrate this. Use of duloxetine in the presence of ethanol may potentiate the deleterious effect of ethanol on the liver." The FDA also recommended "routine blood pressure monitoring" at the new highest recommended dose of 120 mg, "where 24% patients had one or more blood pressure readings of 140/90 vs. 9% of placebo patients."
After the manufacturing issues were resolved, the liver toxicity warning included in the prescribing information, and the follow-up studies showed that duloxetine does not cause QTc interval prolongation, duloxetine was approved by the FDA for depression and diabetic neuropathy in 2004. In 2007, Health Canada approved duloxetine for the treatment of depression and diabetic peripheral neuropathic pain.
Duloxetine was approved for use of stress urinary incontinence (SUI) in the EU in 2004. In 2005, Lilly withdrew the duloxetine application for stress urinary incontinence (SUI) in the U.S., stating that discussions with the FDA indicated "the agency is not prepared at this time to grant approval ... based on the data package submitted." A year later Lilly abandoned the pursuit of this indication in the U.S. market.
The FDA approved duloxetine for the treatment of generalized anxiety disorder in February 2007.
Cymbalta generated sales of nearly $5 billion in 2012 with $4 billion of that in the U.S., but its patent protection terminated January 1, 2014. Lilly received a six-month extension beyond June 30, 2013 after testing for the treatment of depression in adolescents, which may produce $1.5 billion in added sales. It was the most prescribed antidepressant in 2013–14.
The first generic duloxetine was marketed by Dr. Reddy.
References
- ^ Drugs.com International duloxetine brands Page accessed March 10, 2016
- "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Duloxetine". Monograph. The American Society of Health-System Pharmacists. Retrieved 2015-02-26.
- "MeSH Browser". meshb.nlm.nih.gov. Retrieved 2016-11-28.
- National Institute for Health and Clinical Excellence. Clinical guideline 96: Neuropathic pain – pharmacological management. London, 2010.
- Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D (May 2011). "Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation". Neurology. 76 (20): 1758–65. doi:10.1212/WNL.0b013e3182166ebe. PMC 3100130. PMID 21482920.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - "Cymbalta (duloxetine hydrochloride) Delayed-Release Capsules for Oral Use" (PDF).
- Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL (June 2014). "Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline". Journal of Clinical Oncology. 32 (18): 1941–67. doi:10.1200/JCO.2013.54.0914. PMID 24733808.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - Sommer C, Häuser W, Alten R, Petzke F, Späth M, Tölle T, Uçeyler N, Winkelmann A, Winter E, Bär KJ (June 2012). "". Schmerz (in German). 26 (3): 297–310. doi:10.1007/s00482-012-1172-2. PMID 22760463.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - Bril V, England JD, Franklin GM, Backonja M, Cohen JA, Del Toro DR, Feldman EL, Iverson DJ, Perkins B, Russell JW, Zochodne DW (June 2011). "Evidence-based guideline: treatment of painful diabetic neuropathy--report of the American Association of Neuromuscular and Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine & Rehabilitation". Muscle & Nerve. 43 (6): 910–7. doi:10.1002/mus.22092. PMC 3100130. PMID 21484835.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, Nurmikko T (September 2010). "EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision". European Journal of Neurology. 17 (9): 1113-e88. doi:10.1111/j.1468-1331.2010.02999.x. PMID 20402746.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Lunn MP, Hughes RA, Wiffen PJ (January 2014). "Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia". The Cochrane Database of Systematic Reviews. 1 (1): CD007115. doi:10.1002/14651858.CD007115.pub3. PMID 24385423.
- ^ "Towards better patient care: drugs to avoid in 2014". Prescrire International. 23 (150): 161–5. June 2014. PMID 25121155.
- Cipriani A, Koesters M, Furukawa TA, Nosè M, Purgato M, Omori IM, Trespidi C, Barbui C (October 2012). "Duloxetine versus other anti-depressive agents for depression". The Cochrane Database of Systematic Reviews. 10: CD006533. doi:10.1002/14651858.cd006533.pub2. PMC 4169791. PMID 23076926.
- Swiatek, Jeff (2013-10-13). "Loss of Cymbalta patent a major blow for Eli Lilly". Indianapolis Star. Retrieved 2015-02-27.
- Carter NJ, McCormack PL (2009). "Duloxetine: a review of its use in the treatment of generalized anxiety disorder". CNS Drugs. 23 (6): 523–41. doi:10.2165/00023210-200923060-00006. PMID 19480470.
- Kerwin, Robert; Taylor, David H.; Carol Paton (2007). Maudsley Prescribing Guidelines. Informa Healthcare. p. 254. ISBN 0-415-42416-X.
- "Clinical practice guidelines. Management of anxiety disorders". Canadian Journal of Psychiatry. Revue Canadienne De Psychiatrie. 51 (8 Suppl 2): 9S–91S. July 2006. PMID 16933543.
- Patel G, Fancher TL (December 2013). "In the clinic. Generalized anxiety disorder". Annals of Internal Medicine. 159 (11): ITC6–1, ITC6–2, ITC6–3, ITC6–4, ITC6–5, ITC6–6, ITC6–7, ITC6–8, ITC6–9, ITC6–10, ITC6–11, quiz ITC6–12. doi:10.7326/0003-4819-159-11-201312030-01006. PMID 24297210.
- ^ Josefberg H (2004-09-03). "Application number 21-733. Medical review(s)" (PDF). FDA. Retrieved 2009-04-14.
- Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S (July 2005). "Duloxetine vs. placebo in patients with painful diabetic neuropathy". Pain. 116 (1–2): 109–18. doi:10.1016/j.pain.2005.03.029. PMID 15927394.
- Raskin J, Pritchett YL, Wang F, D'Souza DN, Waninger AL, Iyengar S, Wernicke JF (2005). "A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain". Pain Medicine. 6 (5): 346–56. doi:10.1111/j.1526-4637.2005.00061.x. PMID 16266355.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - Wong MC, Chung JW, Wong TK (July 2007). "Effects of treatments for symptoms of painful diabetic neuropathy: systematic review". BMJ. 335 (7610): 87. doi:10.1136/bmj.39213.565972.AE. PMC 1914460. PMID 17562735.
- "Duloxetine: new indication. Depression and diabetic neuropathy: too many adverse effects". Prescrire International. 15 (85): 168–72. October 2006. PMID 17121211.
- Sultan A, Gaskell H, Derry S, Moore RA (August 2008). "Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials". BMC Neurology. 8: 29. doi:10.1186/1471-2377-8-29. PMC 2529342. PMID 18673529.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Acuna C (October 2008). "Duloxetine for the treatment of fibromyalgia". Drugs of Today. 44 (10): 725–34. doi:10.1358/dot.2008.44.10.1269675. PMID 19137126.
- "FDA Approves Cymbalta for the Management of Fibromyalgia". Eli Lilly Co. 2008-06-16. Retrieved 2008-06-17.
- Citrome L, Weiss-Citrome A (January 2012). "A systematic review of duloxetine for osteoarthritic pain: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed?". Postgraduate Medicine. 124 (1): 83–93. doi:10.3810/pgm.2012.01.2521. PMID 22314118.
- Myers J, Wielage RC, Han B, Price K, Gahn J, Paget MA, Happich M (March 2014). "The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis". BMC Musculoskeletal Disorders. 15: 76. doi:10.1186/1471-2474-15-76. PMC 4007556. PMID 24618328.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - "FDA clears Cymbalta to treat chronic musculoskeletal pain". FDA Press Announcements. Food and Drug Administration. 4 November 2010. Retrieved 19 August 2013.
- National Institute for Health and Clinical Excellence. Clinical guideline 40: Urinary incontinence. London, 2006.
- Maund E, Guski LS, Gøtzsche PC (February 2017). "Considering benefits and harms of duloxetine for treatment of stress urinary incontinence: a meta-analysis of clinical study reports". CMAJ. 189 (5): E194–E203. doi:10.1503/cmaj.151104. PMC 5289870. PMID 28246265.
- Li J, Yang L, Pu C, Tang Y, Yun H, Han P (June 2013). "The role of duloxetine in stress urinary incontinence: a systematic review and meta-analysis". International Urology and Nephrology. 45 (3): 679–86. doi:10.1007/s11255-013-0410-6. PMID 23504618.
- "www.uroweb.org" (PDF). Archived from the original (PDF) on 2014-05-04.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - "Urinary incontinence Introduction CG171". Archived from the original on 2014-05-04.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - "Eli Lilly and Company".
- Report a Serious Problem (2013-08-14). "Information for Healthcare Professionals: Duloxetine (marketed as Cymbalta) – Selective Serotonin Reuptake Inhibitors (SSRIs) or Selective Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) and 5-Hydroxytryptamine Receptor Agonists (Triptans)". Fda.gov. Retrieved 2013-09-18.
- Cymbalta package insert. Indianapolis, IN: Eli Lilly Pharmaceuticals; 2004, September.
- Perahia DG, Kajdasz DK, Walker DJ, Raskin J, Tylee A (May 2006). "Duloxetine 60 mg once daily in the treatment of milder major depressive disorder". International Journal of Clinical Practice. 60 (5): 613–20. doi:10.1111/j.1368-5031.2006.00956.x. PMC 1473178. PMID 16700869.
- Chappell AS, Littlejohn G, Kajdasz DK, Scheinberg M, D'Souza DN, Moldofsky H (June 2009). "A 1-year safety and efficacy study of duloxetine in patients with fibromyalgia". The Clinical Journal of Pain. 25 (5): 365–75. doi:10.1097/ajp.0b013e31819be587. PMID 19454869.
- ^ "Cymbalta - FDA prescribing information, side effects and uses". Drugs.com. Retrieved 2018-09-14.
- Nelson JC, Lu Pritchett Y, Martynov O, Yu JY, Mallinckrodt CH, Detke MJ (2006). "The safety and tolerability of duloxetine compared with paroxetine and placebo: a pooled analysis of 4 clinical trials". Primary Care Companion to the Journal of Clinical Psychiatry. 8 (4): 212–9. PMC 1557468. PMID 16964316.
- ^ Clayton A, Kornstein S, Prakash A, Mallinckrodt C, Wohlreich M (July 2007). "Changes in sexual functioning associated with duloxetine, escitalopram, and placebo in the treatment of patients with major depressive disorder". The Journal of Sexual Medicine. 4 (4 Pt 1): 917–29. doi:10.1111/j.1743-6109.2007.00520.x. PMID 17627739.
- Dueñas H, Brnabic AJ, Lee A, Montejo AL, Prakash S, Casimiro-Querubin ML, Khaled M, Dossenbach M, Raskin J (November 2011). "Treatment-emergent sexual dysfunction with SSRIs and duloxetine: effectiveness and functional outcomes over a 6-month observational period". International Journal of Psychiatry in Clinical Practice. 15 (4): 242–54. doi:10.3109/13651501.2011.590209. PMID 22121997.
- Perahia DG, Kajdasz DK, Desaiah D, Haddad PM (December 2005). "Symptoms following abrupt discontinuation of duloxetine treatment in patients with major depressive disorder". Journal of Affective Disorders. 89 (1–3): 207–12. doi:10.1016/j.jad.2005.09.003. PMID 16266753.
- Levenson M, Holland C. "Antidepressants and Suicidality in Adults: Statistical Evaluation. (Presentation at Psychopharmacologic Drugs Advisory Committee; December 13, 2006)". Retrieved 2007-05-13.
- Stone MB, Jones ML (2006-11-17). "Clinical review: relationship between antidepressant drugs and suicidality in adults" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA. pp. 11–74. Retrieved 2007-09-22.
- Levenson M, Holland C (2006-11-17). "Statistical Evaluation of Suicidality in Adults Treated with Antidepressants" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA. pp. 75–140. Retrieved 2007-09-22.
- "Historical Information on Duloxetine hydrochloride (marketed as Cymbalta)".
- Archived 2008-09-12 at the Wayback Machine Duloxetine Side Effects, and Drug Interactions – RxList Monographs
- Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, Shaw JL, Thompson L, Nelson DL, Hemrick-Luecke SK, Wong DT (December 2001). "Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors". Neuropsychopharmacology. 25 (6): 871–80. doi:10.1016/S0893-133X(01)00298-6. PMID 11750180.

- Stahl, S. (2013). Stahl's essential pharmacology, 4th ed. Cambridge University Press, New York. p. 305, 308, 309.
- Stahl SM, Grady MM, Moret C, Briley M (September 2005). "SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants". CNS Spectrums. 10 (9): 732–47. PMID 16142213.
- ^ Bymaster FP, Lee TC, Knadler MP, Detke MJ, Iyengar S (2005). "The dual transporter inhibitor duloxetine: a review of its preclinical pharmacology, pharmacokinetic profile, and clinical results in depression". Current Pharmaceutical Design. 11 (12): 1475–93. doi:10.2174/1381612053764805. PMID 15892657.
- De Berardis D, Conti CM, Serroni N, Moschetta FS, Olivieri L, Carano A, Salerno RM, Cavuto M, Farina B, Alessandrini M, Janiri L, Pozzi G, Di Giannantonio M (2010). "The effect of newer serotonin-noradrenalin antidepressants on cytokine production: a review of the current literature". International Journal of Immunopathology and Pharmacology. 23 (2): 417–22. doi:10.1177/039463201002300204. PMID 20646337.
- Wang SY, Calderon J, Kuo Wang G (September 2010). "Block of neuronal Na+ channels by antidepressant duloxetine in a state-dependent manner". Anesthesiology. 113 (3): 655–65. doi:10.1097/ALN.0b013e3181e89a93. PMID 20693878.
- ^ "Cymbalta product insert" (PDF).
- Robertson DW, Wong DT, Krushinski JH (1990-09-11). "United States Patent 4,956,388: 3-Aryloxy-3-substituted propanamines". USPTO. Retrieved 2008-05-17.
- Wong DT, Robertson DW, Bymaster FP, Krushinski JH, Reid LR (1988). "LY227942, an inhibitor of serotonin and norepinephrine uptake: biochemical pharmacology of a potential antidepressant drug". Life Sciences. 43 (24): 2049–57. doi:10.1016/0024-3205(88)90579-6. PMID 2850421.
- Bymaster FP, Beedle EE, Findlay J, Gallagher PT, Krushinski JH, Mitchell S, Robertson DW, Thompson DC, Wallace L, Wong DT (December 2003). "Duloxetine (Cymbalta), a dual inhibitor of serotonin and norepinephrine reuptake". Bioorganic & Medicinal Chemistry Letters. 13 (24): 4477–80. doi:10.1016/j.bmcl.2003.08.079. PMID 14643350.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - "Approval package for: application number NDA 721-427. Administrative/Correspondence #2" (PDF). The FDA Center for Drug Evaluation and Research. 2003. Retrieved 2008-05-18.
- FDA news
- "Summary Basis of Decision (SBD): Cymbalta". Health Canada. 2008-05-05. Archived from the original on 2015-03-01. Retrieved 2015-02-27.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - Steyer R (2006-02-15). "Lilly Won't Pursue Yentreve for U.S." TheStreet.com. Archived from the original on 2009-02-02. Retrieved 2008-05-18.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - Lenzer J (July 2005). "FDA warns that antidepressants may increase suicidality in adults". BMJ. 331 (7508): 70. doi:10.1136/bmj.331.7508.70-b. PMC 558648. PMID 16002878.
- "FDA approves antidepressant Cymbalta (duloxetine HCl) for treatment of generalized anxiety disorder". News-Medical. February 26, 2007. Retrieved 25 December 2013.
- Staton, Tracy (July 9, 2012). "Lilly could net $1.5B-plus from Cymbalta extension". FiercePharma. Retrieved 25 December 2013.
- Palmer, Eric (April 11, 2013). "Eli Lilly to lay off hundreds in sales as Cymbalta nears edge of patent cliff". FiercePharma. Retrieved 25 December 2013.
- Hrenchir, Tim (2 September 2015). "10 Most-Prescribed Antidepressant Medications". Newsmax.
- Anson, Pat (December 12, 2013). "Generic Cheaper Versions of Cymbalta Approved". National Pain Report. Retrieved January 2, 2014.
External links
- Eli Lilly Cymbalta Prescribing Guide
- U.S. National Library of Medicine: Drug Information Portal – Duloxetine
| Neuropathic pain and fibromyalgia pharmacotherapies | |
|---|---|
| Monoaminergics |
|
| Ion channel blockers |
|
| Others |
|
| Monoamine reuptake inhibitors | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DATTooltip Dopamine transporter (DRIsTooltip Dopamine reuptake inhibitors) |
| ||||||||||||||
| NETTooltip Norepinephrine transporter (NRIsTooltip Norepinephrine reuptake inhibitors) |
| ||||||||||||||
| SERTTooltip Serotonin transporter (SRIsTooltip Serotonin reuptake inhibitors) |
| ||||||||||||||
| VMATsTooltip Vesicular monoamine transporters | |||||||||||||||
| Others |
| ||||||||||||||
| See also: Receptor/signaling modulators • Monoamine releasing agents • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||