This is an old revision of this page, as edited by Artman40 (talk | contribs) at 15:40, 4 January 2007 (→Coenzymes: NADH is a cofactor, not a coenzyme.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:40, 4 January 2007 by Artman40 (talk | contribs) (→Coenzymes: NADH is a cofactor, not a coenzyme.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
Enzymes are proteins that catalyze (i.e. accelerate) chemical reactions. In these reactions, the molecules at the beginning of the process are called substrates, and the enzyme converts these into different molecules, the products. Almost all processes in the cell need enzymes in order to occur at significant rates. Since enzymes are extremely selective for their substrates and speed up only a few reactions from among many possibilities, the set of enzymes made in a cell determines which metabolic pathways occur in that cell.
Like all catalysts, enzymes work by lowering the activation energy (ΔG) for a reaction, thus dramatically accelerating the rate of the reaction. Most enzyme reaction rates are millions of times faster than those of comparable uncatalyzed reactions. As with all catalysts, enzymes are not consumed by the reactions they catalyze, nor do they alter the equilibrium of these reactions. However, enzymes do differ from most other catalysts by being much more specific. Enzymes are known to catalyze about 4,000 biochemical reactions. Not all biochemical catalysts are proteins, since some RNA molecules called ribozymes also catalyze reactions.
Enzyme activity can be affected by other molecules. Inhibitors are molecules that decrease enzyme activity; activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. Activity is also affected by temperature, pH, and the concentration of substrate. Some enzymes are used commercially, for example, in the synthesis of antibiotics. In addition, some household products use enzymes to speed up biochemical reactions (e.g., enzymes in biological washing powders break down protein or fat stains on clothes; enzymes in meat tenderizers break down proteins, making the meat easier to chew).
Etymology and history

As early as the late 1700s and early 1800s, the digestion of meat by stomach secretions and the conversion of starch to sugars by plant extracts and saliva were known. However, the mechanism by which this occurred had not been identified.
In the 19th century, when studying the fermentation of sugar to alcohol by yeast, Louis Pasteur came to the conclusion that this fermentation was catalyzed by a vital force contained within the yeast cells called "ferments", which were thought to function only within living organisms. He wrote that "alcoholic fermentation is an act correlated with the life and organisation of the yeast cells, not with the death or putrefaction of the cells."
In 1878 German physiologist Wilhelm Kühne (1837–1900) coined the term enzyme, which comes from Greek ενζυμον "in leaven", to describe this process. The word enzyme was used later to refer to nonliving substances such as pepsin, and the word ferment used to refer to chemical activity produced by living organisms.
In 1897 Eduard Buchner began to study the ability of yeast extracts to ferment sugar despite the absence of living yeast cells. In a series of experiments at the University of Berlin, he found that the sugar was fermented even when there were no living yeast cells in the mixture. He named the enzyme that brought about the fermentation of sucrose "zymase". In 1907 he received the Nobel Prize in Chemistry "for his biochemical research and his discovery of cell-free fermentation". Following Buchner's example; enzymes are usually named according to the reaction they carry out. Typically the suffix -ase is added to the name of the substrate (e.g., lactase is the enzyme that cleaves lactose) or the type of reaction (e.g., DNA polymerase forms DNA polymers).
Having shown that enzymes could function outside a living cell, the next step was to determine their biochemical nature. Many early workers noted that enzymatic activity was associated with proteins, but several scientists (such as Nobel laureate Richard Willstätter) argued that proteins were merely carriers for the true enzymes and that proteins per se were incapable of catalysis. However, in 1926, James B. Sumner showed that the enzyme urease was a pure protein and crystallized it; Sumner did likewise for the enzyme catalase in 1937. The conclusion that pure proteins can be enzymes was definitively proved by Northrop and Stanley, who worked on the digestive enzymes pepsin (1930), trypsin and chymotrypsin. These three scientists were awarded the 1946 Nobel Prize in Chemistry.
This discovery that enzymes could be crystalised eventually allowed their structures to be solved by x-ray crystallography. This was first done for lysozyme, an enzyme found in tears, saliva and egg whites that digests the coating of some bacteria; the structure was solved by a group led by David Chilton Phillips and published in 1965. This high-resolution structure of lysozyme marked the beginning of the field of structural biology and the effort to understand how enzymes work at an atomic level of detail.
Structures and mechanisms
See also: Enzyme catalysis
The activities of enzymes are determined by their three-dimensional structure.
Most enzymes are much larger than the substrates they act on, and only a very small portion of the enzyme (around 3–4 amino acids) is directly involved in catalysis. The region that contains these catalytic residues, binds the substrate, and then carries out the reaction is known as the active site. Enzymes can also contain sites that bind cofactors, which are needed for catalysis. Some enzymes also have binding sites for small molecules, which are often direct or indirect products or substrates of the reaction catalyzed. This binding can serve to increase or decrease the enzyme's activity, providing a means for feedback regulation.
Like all proteins, enzymes are made as long, linear chains of amino acids that fold to produce a three-dimensional product. Each unique amino acid sequence produces a unique structure, which has unique properties. Individual protein chains may sometimes group together to form a protein complex. Most enzymes can be denatured—that is, unfolded and inactivated—by heating, which destroys the three-dimensional structure of the protein. Depending on the enzyme, denaturation may be reversible or irreversible.
Specificity
Enzymes are usually very specific as to which reactions they catalyze and the substrates that are involved in these reactions. Complementary shape, charge and hydrophilic/hydrophobic characteristics of enzymes and substrates are responsible for this specificity. Enzymes can also show impressive levels of stereospecificity, regioselectivity and chemoselectivity.
Some of the enzymes showing the highest specificity and accuracy are involved in the copying and expression of the genome. These enzymes have "proof-reading" mechanisms. Here, an enzyme such as DNA polymerase catalyses a reaction in a first step and then checks that the product is correct in a second step. This two-step process results in average error rates of less than 1 error in 100 million reactions in high-fidelity mammalian polymerases. Similar proofreading mechanisms are also found in aminoacyl tRNA synthetases and ribosomes.
Some enzymes that produce secondary metabolites are described as promiscuous, as they can act on a relatively broad range of different substrates. It has been suggested that this broad substrate specificity is important for the evolution of new biosynthetic pathways.
"Lock and key" model
Enzymes are very specific, and it was suggested by Emil Fischer in 1894 that this was because both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. This is often referred to as "the lock and key" model. However, while this model explains enzyme specificity, it fails to explain the stabilization of the transition state that enzymes achieve.
Induced fit model

In 1958 Daniel Koshland suggested a modification to the lock and key model: since enzymes are rather flexible structures, the active site can be reshaped by interactions with the substrate as the substrate interacts with the enzyme. As a result, the amino acid side chains which make up the active site are molded into the precise positions that enable the enzyme to perform its catalytic function. In some cases, such as glycosidases, the substrate molecule also changes shape slightly as it enters the active site.
Mechanisms
Enzymes can act in several ways, all of which lower ΔG:
- Lowering the activation energy by creating an environment in which the transition state is stabilised (e.g. straining the shape of a substrate - by binding the transition-state conformation of the substrate/product molecules, the enzyme distorts the bound substrate(s) into their transition state form, thereby reducing the amount of energy required to complete the transition).
- Providing an alternative pathway (e.g. temporarily reacting with the substrate to form an intermediate which would be impossible in the absence of the enzyme).
- Reducing the reaction entropy change by bringing substrates together in the correct orientation to react. Considering ΔH alone overlooks this effect.
Dynamics and function
Recent investigations have provided new insights into the connection between internal dynamics of enzymes and their mechanism of catalysis. An enzyme's internal dynamics are described as the movement of internal parts (e.g. amino acids, a group of amino acids, a loop region, an alpha helix, neighboring beta-sheets or even entire domain) of these biomolecules, which can occur at various time-scales ranging from femtoseconds to seconds. Networks of protein residues throughout an enzyme's structure can contribute to catalysis through dynamic motions. Protein motions are vital to many enzymes, but whether small and fast vibrations or larger and slower conformational movements are more important depends on the type of reaction involved. These new insights also have implications in understanding allosteric effects, producing designer enzymes and developing new drugs.
Allosteric modulation
Allosteric enzymes change their structure in response to binding of effectors. Modulation can be direct, where the effector binds directly to binding sites in the enzyme, or indirect, where the effector binds to other proteins or protein subunits that interact with the allosteric enzyme and thus influence catalytic activity.
Cofactors and coenzymes
Main articles: Cofactor (biochemistry) and CoenzymeCofactors
Some enzymes do not need any additional components to show full activity. However, others require non-protein molecules to be bound for activity. Cofactors can be either inorganic (e.g., metal ions and iron-sulfur clusters) or organic compounds, (e.g., flavin and heme). Organic cofactors (coenzymes) are usually prosthetic groups, which are tightly bound to the enzymes that they assist. These tightly-bound cofactors are distinguished from other coenzymes, such as NADH, since they are not released from the active site during the reaction.
An example of an enzyme that contains a cofactor is carbonic anhydrase, and is shown in the ribbon diagram above with a zinc cofactor bound in its active site. These tightly-bound molecules are usually found in the active site and are involved in catalysis. For example, flavin and heme cofactors are often involved in redox reactions.
Enzymes that require a cofactor but do not have one bound are called apoenzymes. An apoenzyme together with its cofactor(s) is called a holoenzyme (i.e., the active form). Most cofactors are not covalently attached to an enzyme, but are very tightly bound. However, organic prosthetic groups can be covalently bound (e.g., thiamine pyrophosphate in the enzyme pyruvate dehydrogenase).
Coenzymes
Coenzymes are small molecules that transport chemical groups from one enzyme to another. Some of these chemicals such as riboflavin, thiamine and folic acid are vitamins, this is when these compounds cannot be made in the body and must be acquired from the diet. The chemical groups carried include the hydride ion (H+ + 2e-) carried by NAD or NADP, the acetyl group carried by coenzyme A, formyl, methenyl or methyl groups carried by folic acid and the methyl group carried by S-adenosylmethionine.
Since coenzymes are chemically changed as a consequence of enzyme action, it is useful to consider coenzymes to be a special class of substrates, or second substrates, which are common to many different enzymes. For example, about 700 enzymes are known to use the cofactor NADH.
Coenzymes are usually regenerated and their concentrations maintained at a steady level inside the cell: for example, NADPH is regenerated through the pentose phosphate pathway and S-adenosylmethionine by methionine adenosyltransferase.
Thermodynamics
Main articles: Activation energy, Thermodynamic equilibrium, and Chemical equilibrium
As all catalysts, enzymes do not alter the position of the chemical equilibrium of the reaction. Usually, in the presence of an enzyme, the reaction runs in the same direction as it would without the enzyme, just more quickly. However, in the absence of the enzyme, other possible uncatalyzed, "spontaneous" reactions might lead to different products, because in those conditions this different product is formed faster.
Furthermore, enzymes can couple two or more reactions, so that a thermodynamically favorable reaction can be used to "drive" a thermodynamically unfavorable one. For example, the hydrolysis of ATP is often used to drive other chemical reactions.
Enzymes catalyze the forward and backward reactions equally. They do not alter the equilibrium itself, but only the speed at which it is reached. For example, carbonic anhydrase catalyzes its reaction in either direction depending on the concentration of its reactants.
Nevertheless, if the equilibrium is greatly displaced in one direction, that is, in a very exergonic reaction, the reaction is effectively irreversible. Under these conditions the enzyme will, in fact, only catalyze the reaction in the thermodynamically allowed direction.
Kinetics
Main article: Enzyme kinetics
Enzyme kinetics is the investigation of how enzymes bind substrates and turn them into products. The rate data used in kinetic analyses are obtained from enzyme assays. In 1913 Leonor Michaelis and Maud Menten proposed a quantitative theory of enzyme kinetics, which is referred to as Michaelis-Menten kinetics. Their work was further developed by G. E. Briggs and J. B. S. Haldane, who derived kinetic equations that are still widely used today.
The major contribution of Michaelis and Menten was to think of enzyme reactions in two stages. In the first, the substrate binds reversibly to the enzyme, forming the enzyme-substrate complex. This is sometimes called the Michaelis-Menten complex in their honor. The enzyme then catalyzes the chemical step in the reaction and releases the product.
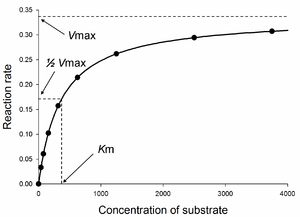
Enzymes can catalyze up to several million reactions per second. For example, the reaction catalysed by orotidine 5'-phosphate decarboxylase will consume half of its substrate in 78 million years if no enzyme is present. However, when the decarboxylase is added, the same process takes just 25 milliseconds. Enzyme rates depend on solution conditions and substrate concentration. Conditions that denature the protein abolish enzyme activity, such as high temperatures, extremes of pH or high salt concentrations, while raising substrate concentration tends to increase activity. To find the maximum speed of an enzymatic reaction, the substrate concentration is increased until a constant rate of product formation is seen. This is shown in the saturation curve, shown on the right. Saturation happens because, as substrate concentration increases, more and more of the free enzyme is converted into the substrate-bound ES form. At the maximum velocity (Vmax) of the enzyme, all enzyme active sites are saturated with substrate, and the amount of ES complex is the same as the total amount of enzyme.
However, Vmax is only one kinetic constant of enzymes. The amount of substrate needed to achieve a given rate of reaction is also important. This is given by the Michaelis-Menten constant (Km), which is the substrate concentration required for an enzyme to reach one-half its maximum velocity. Each enzyme has a characteristic Km for a given substrate, and this can show how tight the binding of the substrate is to the enzyme. Another useful constant is kcat, which is the number of substrate molecules handled by one active site per second.
The efficiency of an enzyme can be expressed in terms of kcat/Km. This is also called the specificity constant and incorporates the rate constants for all steps in the reaction. Because the specificity constant reflects both affinity and catalytic ability, it is useful for comparing different enzymes against each other, or the same enzyme with different substrates. The theoretical maximum for the specificity constant is called the diffusion limit and is about 10 to 10 (M s). At this point every collision of the enzyme with its substrate will result in catalysis, and the rate of product formation is not limited by the reaction rate but by the diffusion rate. Enzymes with this property are called catalytically perfect or kinetically perfect. Example of such enzymes are triose-phosphate isomerase, carbonic anhydrase, acetylcholinesterase, catalase, fumarase, ß-lactamase, and superoxide dismutase.
Some enzymes operate with kinetics which are faster than diffusion rates, which would seem to be impossible. Several mechanisms have been invoked to explain this phenomenon. Some proteins are believed to accelerate catalysis by drawing their substrate in and pre-orienting them by using dipolar electric fields. Other models invoke a quantum-mechanical tunneling explanation, whereby a proton or an electron can tunnel through activation barriers, although for proton tunneling this model remains somewhat controversial. Quantum tunneling for protons has been observed in tryptamine. This suggests that enzyme catalysis may be more accurately characterized as "through the barrier" rather than the traditional model, which requires substrates to go "over" a lowered energy barrier.
Inhibition
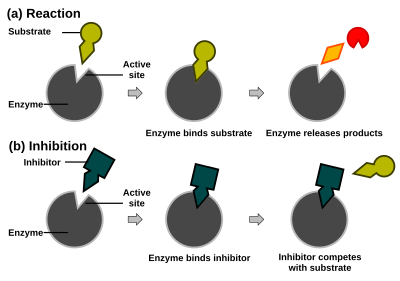
Enzyme reaction rates can be decreased by various types of enzyme inhibitors.
Reversible inhibitors
Competitive inhibition
In competitive inhibition the inhibitor binds to the substrate binding site (figure right, top, thus preventing substrate from binding (EI complex). Often competitive inhibitors strongly resemble the real substrate of the enzyme. For example, methotrexate is a competitive inhibitor of the enzyme dihydrofolate reductase, which catalyzes the reduction of dihydrofolate to tetrahydrofolate. The similarity between the structures of folic acid and this drug are shown in the figure to the right bottom.
Non-competitive inhibition
Non-competitive inhibitors can bind either to the active site, or to other parts of the enzyme far away from the substrate-binding site. Moreover, non-competitive inhibitors bind to the enzyme-substrate (ES) complex and to the free enzyme. Their binding to this site changes the shape of the enzyme and stops the active site binding substrate(s). Consequently, since there is no direct competition between the substrate and inhibitor for the enzyme, the extent of inhibition depends only on the inhibitor concentration and will not be affected by the substrate concentration.
Irreversible inhibitors
Some enzyme inhibitors react with the enzyme and form a covalent adduct with the protein. The inactivation produced by this type of inhibitor cannot be reversed. A class of these compounds called suicide inhibitors includes eflornithine a drug used to treat the parasitic disease sleeping sickness.
Uses of inhibitors
Inhibitors are often used as drugs, but they can also act as poisons. However, the difference between a drug and a poison is usually only a matter of amount, since most drugs are toxic at some level, as Paracelsus wrote, "In all things there is a poison, and there is nothing without a poison." Equally, antibiotics and other anti-infective drugs are just specific poisons that can kill a pathogen but not its host.
An example of an inhibitor being used as a drug is aspirin, which inhibits the COX-1 and COX-2 enzymes that produce the inflammation messenger prostaglandin, thus suppressing pain and inflammation. The poison cyanide is an irreversible enzyme inhibitor that combines with the copper and iron in the active site of the enzyme cytochrome c oxidase and blocks cellular respiration.
In many organisms inhibitors may act as part of a feedback mechanism. If an enzyme produces too much of one substance in the organism, that substance may act as an inhibitor for the enzyme that produces it, causing production of the substance to slow down or stop when there is sufficient amount. This is a form of negative feedback.
Biological function
Enzymes serve a wide variety of functions inside living organisms. They are indispensable for signal transduction and cell regulation, often via kinases and phosphatases. They also generate movement, with myosin hydrolysing ATP to generate muscle contraction and also moving cargo around the cell as part of the cytoskeleton. Other ATPases in the cell membrane are ion pumps involved in active transport. Enzymes are also involved in more exotic functions, such as luciferase generating light in fireflies.
Viruses can contain enzymes for infecting cells, such as the HIV integrase and reverse transcriptase, or for viral release from cells, like the influenza virus neuraminidase.
An important function of enzymes is in the digestive systems of animals. Enzymes such as amylases and proteases break down large molecules (starch or proteins, respectively) into smaller ones, so they can be absorbed by the intestines. Starch is inabsorbable in the intestine but enzymes hydrolyse the starch chains into smaller molecules such as maltose and eventually glucose, which can then be absorbed. Different enzymes digest different food substances. In ruminants which have a herbivorous diets, bacteria in the gut produce another enzyme, cellulase to break down the cellulose cell walls of plant fiber.
Several enzymes can work together in a specific order, creating metabolic pathways. In a metabolic pathway, one enzyme takes the product of another enzyme as a substrate. After the catalytic reaction, the product is then passed on to another enzyme. Sometimes more than one enzyme can catalyse the same reaction in parallel, this can allow more complex regulation: with for example a low contant activity being provided by one enzyme but an inducible high activity from a second enzyme.
Enzymes determine what steps occur in these pathways. Without enzymes, metabolism would neither progress through the same steps, nor be fast enough to serve the needs of the cell. Indeed, a metabolic pathway such as glycolysis could not exist independently of enzymes. Glucose, for example, can react directly with ATP to become phosphorylated at one or more of its carbons. However, if hexokinase is present, glucose-6-phosphate is the only product, as this reaction will occur most swiftly. Consequently, the network of metabolic pathways within each cell depends on the set of functional enzymes that are present.
Control of activity
There are five main ways that enzyme activity is controlled in the cell.
- Enzyme production (transcription and translation of enzyme genes) can be enhanced or diminished by a cell in response to changes in the cell's environment. This form of gene regulation is called enzyme induction and inhibition. For example, bacteria may become resistant to antibiotics such as penicillin because enzymes called beta-lactamases are induced that hydrolyse the crucial beta-lactam ring within the penicillin molecule. Another example are enzymes in the liver called cytochrome P450 oxidases, which are important in drug metabolism. Induction or inhibition of these enzymes can cause drug interactions.
- Enzymes can be compartmentalized, with different metabolic pathways occurring in different cellular compartments. For example, fatty acids are synthesized by one set of enzymes in the cytosol, endoplasmic reticulum and the Golgi apparatus and used by a different set of enzymes as a source of energy in the mitochondrion, through β-oxidation.
- Enzymes can be regulated by inhibitors and activators. For example, the end product(s) of a metabolic pathway are often inhibitors for one of the first enzymes of the pathway (usually the first irreversible step, called committed step), thus regulating the amount of end product made by the pathways. Such a regulatory mechanism is called a negative feedback mechanism, because the amount of the end product produced is regulated by its own concentration. Negative feedback mechanism can effectively adjust the rate of synthesis of intermediate metabolites according to the demands of the cells. This helps allocate materials and energy economically, and prevents the manufacture of excess end products. Like other homeostatic devices, the control of enzymatic action helps to maintain a stable internal environment in living organisms.
- Enzymes can be regulated through post-translational modification. This can include phosphorylation, myristoylation and glycosylation. For example, in the response to insulin, the phosphorylation of multiple enzymes, including glycogen synthase, helps control the synthesis or degradation of glycogen and allows the cell to respond to changes in blood sugar. Another example of post-translational modification is the cleavage of the polypeptide chain. Chymotrypsin, a digestive protease, is produced in inactive form as chymotrypsinogen in the pancreas and transported in this form to the stomach where it is activated. This stops the enzyme from digesting the pancreas or other tissues before it enters the gut. This type of inactive precursor to an enzyme is known as a zymogen.
- Some enzymes may become activated when localized to a different environment (eg. from a reducing (cytoplasm) to an oxidising (periplasm) environment, high pH to low pH etc). For example, hemagglutinin of the influenza virus undergoes a conformational change once it encounters the acidic environment of the host cell vesicle causing its activation.
Involvement in disease
Since the tight control of enzyme activity is essential for homeostasis, any malfunction (mutation, overproduction, underproduction or deletion) of a single critical enzyme can lead to a genetic disease. The importance of enzymes is shown by the fact that a lethal illness can be caused by the malfunction of just one type of enzyme out of the thousands of types present in our bodies.
One example is the most common type of phenylketonuria. A mutation of a single amino acid in the enzyme phenylalanine hydroxylase, which catalyzes the first step in the degradation of phenylalanine, results in build-up of phenylalanine and related products. This can lead to mental retardation if the disease is untreated.
Another example is when germline mutations in genes coding for DNA repair enzymes cause hereditary cancer syndromes such as xeroderma pigmentosum. Defects in these enzymes cause cancer since the body is less able to repair mutations in the genome. This causes a slow accumulation of mutations and results in the development of many types of cancer in the sufferer.
Naming conventions
An enzyme's name is often derived from its substrate or the chemical reaction it catalyzes, with the word ending in -ase. Examples are lactase, alcohol dehydrogenase and DNA polymerase. This may result in different enzymes, called isoenzymes, with the same function having the same basic name. Isoenzymes have a different amino acid sequence and might be distinguished by their optimal pH, kinetic properties or immunologically. Furthermore, the normal physiological reaction an enzyme catalyzes may not be the same as under artifical conditions. This can result in the same enzyme being identified with two different names. E.g. Glucose isomerase, used industrially to convert glucose into the sweetener fructose, is a xylose isomerase in vivo.
The International Union of Biochemistry and Molecular Biology have developed a nomenclature for enzymes, the EC numbers; each enzyme is described by a sequence of four numbers preceded by "EC". The first number broadly classifies the enzyme based on its mechanism:
The top-level classification is
- EC 1 Oxidoreductases: catalyze oxidation/reduction reactions
- EC 2 Transferases: transfer a functional group (e.g. a methyl or phosphate group)
- EC 3 Hydrolases: catalyze the hydrolysis of various bonds
- EC 4 Lyases: cleave various bonds by means other than hydrolysis and oxidation
- EC 5 Isomerases: catalyze isomerization changes within a single molecule
- EC 6 Ligases: join two molecules with covalent bonds
The complete nomenclature can be browsed at http://www.chem.qmul.ac.uk/iubmb/enzyme/.
Industrial applications
Enzymes are used in the chemical industry and other industrial applications when extremely specific catalysts are required. However, enzymes in general are limited in the number of reactions they have evolved to catalyse and also by their lack of stability in organic solvents and at high temperatures. Consequently, protein engineering is an active area of research and involves attempts to create new enzymes with novel properties, either through rational design or in vitro evolution.
| Application | Enzymes used | Uses |
Baking industry  |
Fungal alpha-amylase enzymes are normally inactivated at about 50 degrees Celsius, but are destroyed during the baking process. | Catalyze breakdown of starch in the flour to sugar. Yeast action on sugar produces carbon dioxide. Used in production of white bread, buns, and rolls. |
| Proteases | Biscuit manufacturers use them to lower the protein level of flour. | |
| Baby foods | Trypsin | To predigest baby foods. |
Brewing industry  |
Enzymes from barley are released during the mashing stage of beer production. | They degrade starch and proteins to produce simple sugar, amino acids and peptides that are used by yeast for fermentation. |
| Industrially produced barley enzymes | Widely used in the brewing process to substitute for the natural enzymes found in barley. | |
| Amylase, glucanases, proteases | Split polysaccharides and proteins in the malt. | |
| Betaglucosidase | Improve the filtration characteristics. | |
| Amyloglucosidase | Low-calorie beer. | |
| Proteases | Remove cloudiness produced during storage of beers. | |
| Fruit juices | Cellulases, pectinases | Clarify fruit juices |
Dairy industry  |
Rennin, derived from the stomachs of young ruminant animals (like calves and lambs). | Manufacture of cheese, used to hydrolyze protein. |
| Microbially produced enzyme | Now finding increasing use in the dairy industry. | |
| Lipases | Is implemented during the production of Roquefort cheese to enhance the ripening of the blue-mould cheese. | |
| Lactases | Break down lactose to glucose and galactose. | |
| Starch industry | Amylases, amyloglucosideases and glucoamylases | Converts starch into glucose and various syrups. |
| Glucose isomerase | Converts glucose into fructose in production of (high fructose syrups from starchy materials. These syrups have enhanced sweetening properties and lower calorific values) than sucrose for the same level of sweetness. | |
| Meat tenderizers | Papain | To soften meat for cooking. |
| Biological detergent |
Primarily proteases, produced in an extracellular form from bacteria | Used for presoak conditions and direct liquid applications helping with removal of protein stains from clothes. |
| Amylases | Detergents for machine dish washing to remove resistant starch residues. | |
| Lipases | Used to assist in the removal of fatty and oily stains. | |
| Cellulases | Used in biological fabric conditioners. | |
| Contact lens cleaners | Proteases | To remove proteins on contact lens to prevent infections. |
| Rubber industry | Catalase | To generate oxygen from peroxide to convert latex into foam rubber. |
Paper industry |
Amylases, Xylanases, Cellulases and ligninases | Degrade starch to lower viscosity, aiding sizing and coating paper. Xylanases reduce bleach required for decolorising; cellulases smooth fibers, enhance water drainage, and promote ink removal; lipases reduce pitch and lignin-degrading enzymes remove lignin to soften paper. |
| Photographic industry | Protease (ficin) | Dissolve gelatin off scrap film, allowing recovery of its silver content. |
Molecular biology  |
Restriction enzymes, DNA ligase and polymerases | Used to manipulate DNA in genetic engineering, important in pharmacology, agriculture and medicine. Essential for restriction digestion and the polymerase chain reaction. Molecular biology is also important in forensic science. |
See also
References
- Bairoch A. (2000). "The ENZYME database in 2000" (PDF). Nucleic Acids Res. 28: 304–305. PMID 10592255.
- de Réaumur, RAF (1752). "Observations sur la digestion des oiseaux". Histoire de l'academie royale des sciences. 1752: 266, 461.
- Williams, H. S. (1904) A History of Science: in Five Volumes. Volume IV: Modern Development of the Chemical and Biological Sciences Harper and Brothers (New York)
- Dubos J. (1951). "Louis Pasteur: Free Lance of Science, Gollancz. Quoted in Manchester K. L. (1995) Louis Pasteur (1822–1895)--chance and the prepared mind". Trends Biotechnol. 13 (12): 511–515. PMID 8595136.
- Nobel Laureate Biography of Eduard Buchner at http://nobelprize.org
- Text of Eduard Buchner's 1907 Nobel lecture at http://nobelprize.org
- 1946 Nobel prize for Chemistry laureates at http://nobelprize.org
- Blake CC, Koenig DF, Mair GA, North AC, Phillips DC, Sarma VR. (1965). "Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution". Nature. 22 (206): 757–761. PMID 5891407.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Anfinsen C.B. (1973). "Principles that Govern the Folding of Protein Chains". Science: 223–230. PMID 4124164.
- The Catalytic Site Atlas at The European Bioinformatics Institute
- Jaeger KE, Eggert T. (2004). "Enantioselective biocatalysis optimized by directed evolution". Curr Opin Biotechnol. 15(4): 305–313. PMID 15358000.
- Shevelev IV, Hubscher U. (2002). "The 3' 5' exonucleases". Nat Rev Mol Cell Biol. 3 (5): 364–376. PMID 11988770.
- Berg J., Tymoczko J. and Stryer L. (2002) Biochemistry. W. H. Freeman and Company ISBN 0-7167-4955-6
- Ibba M, Soll D. (2000). "Aminoacyl-tRNA synthesis". Annu Rev Biochem. 69: 617–650. PMID 10966471.
- Rodnina MV, Wintermeyer W. (2001). "Fidelity of aminoacyl-tRNA selection on the ribosome: kinetic and structural mechanisms". Annu Rev Biochem. 70: 415–435. PMID 11395413.
- Firn, Richard. "The Screening Hypothesis - a new explanation of secondary product diversity and function". Retrieved 2006-10-11.
- Fischer E. (1894). "Einfluss der Configuration auf die Wirkung der Enzyme". Ber. Dt. Chem. Ges. 27: 2985–2993.
{{cite journal}}: line feed character in|journal=at position 9 (help) - Koshland D. E. (1958). "Application of a Theory of Enzyme Specificity to Protein Synthesis". Proc. Natl. Acad. Sci. 44 (2): 98–104. PMID 16590179.
- Vasella A, Davies GJ, Bohm M. (2002). "Glycosidase mechanisms". Curr Opin Chem Biol. 6 (5): 619–629. PMID 12413546.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Fersht, A (1985) Enzyme Structure and Mechanism (2nd ed) p50-52 W H Freeman & co, New York ISBN 0-7167-1615-1
- Eisenmesser EZ, Bosco DA, Akke M, Kern D. Enzyme dynamics during catalysis. Science. 2002 Feb 22;295(5559):1520-3. PMID: 11859194
- Agarwal PK. Role of protein dynamics in reaction rate enhancement by enzymes. J Am Chem Soc. 2005 Nov 2;127(43):15248-56. PMID: 16248667
- Eisenmesser EZ, Millet O, Labeikovsky W, Korzhnev DM, Wolf-Watz M, Bosco DA, Skalicky JJ, Kay LE, Kern D. Intrinsic dynamics of an enzyme underlies catalysis. Nature. 2005 Nov 3;438(7064):117-21. PMID: 16267559
- Yang LW, Bahar I. (2005). "Coupling between catalytic site and collective dynamics: A requirement for mechanochemical activity of enzymes". Structure. 13: 893–904. PMID 15939021.
{{cite journal}}: Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help) - Agarwal PK, Billeter SR, Rajagopalan PT, Benkovic SJ, Hammes-Schiffer S. (2002). "Network of coupled promoting motions in enzyme catalysis". Proc. Natl. Acad. Sci. U S A. 99: 2794–9. PMID 11867722.
{{cite journal}}: Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Agarwal PK, Geist A, Gorin A. Protein dynamics and enzymatic catalysis: investigating the peptidyl-prolyl cis-trans isomerization activity of cyclophilin A. Biochemistry. 2004 Aug 24;43(33):10605-18. PMID: 15311922
- Tousignant A, Pelletier JN. (2004). "Protein motions promote catalysis". Chem Biol. 11 (8): 1037–42. PMID 15324804.
{{cite journal}}: Unknown parameter|month=ignored (help) - Fisher Z, Hernandez Prada JA, Tu C, Duda D, Yoshioka C, An H, Govindasamy L, Silverman DN and McKenna R. (2005). "Structural and kinetic characterization of active-site histidine as a proton shuttle in catalysis by human carbonic anhydrase II". Biochemistry. 44(4): 1097–115. PMID 15667203.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - AF Wagner, KA Folkers (1975) Vitamins and coenzymes. Interscience Publishers New York| ISBN 0-88275-258-8
- BRENDA The Comprehensive Enzyme Information System
- Michaelis L., Menten M. (1913). "Die Kinetik der Invertinwirkung". Biochem. Z. 49: 333–369.
- Briggs G. E., Haldane J. B. S. (1925). "A note on the kinetics of enzyme action". Biochem. J. 19: 339–339. PMID 16743508.
- Radzicka A, Wolfenden R. (1995). "A proficient enzyme". Science. 6 (267): 90–931. PMID 7809611.
- Garcia-Viloca M., Gao J., Karplus M., Truhlar D. G. (2004). "How enzymes work: analysis by modern rate theory and computer simulations". Science. 303 (5655): 186–195. PMID 14716003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Olsson M. H., Siegbahn P. E., Warshel A. (2004). "Simulations of the large kinetic isotope effect and the temperature dependence of the hydrogen atom transfer in lipoxygenase". J. Am. Chem. Soc. 126 (9): 2820–1828. PMID 14995199.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Masgrau L., Roujeinikova A., Johannissen L. O., Hothi P., Basran J., Ranaghan K. E., Mulholland A. J., Sutcliffe M. J., Scrutton N. S., Leys D. (2006). "Atomic Description of an Enzyme Reaction Dominated by Proton Tunneling". Science. 312 (5771): 237–241. PMID 16614214.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Ball, Philip (2006) The Devil's Doctor: Paracelsus and the World of Renaissance Magic and Science. Farrar, Straus and Giroux ISBN 0-374-22979-1
- Yoshikawa S and Caughey WS. (1990). "Infrared evidence of cyanide binding to iron and copper sites in bovine heart cytochrome c oxidase. Implications regarding oxygen reduction". J Biol Chem. 265 (14): 7945–7958. PMID 2159465.
{{cite journal}}: Unknown parameter|month=ignored (help) - Hunter T. (1995). "Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling". Cell. 80(2): 225–236. PMID 7834742.
- Berg JS, Powell BC, Cheney RE. (2001). "A millennial myosin census". Mol Biol Cell. 12(4): 780–794. PMID 11294886.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Meighen EA. (1991). "Molecular biology of bacterial bioluminescence". Microbiol Rev. 55(1): 123–142. PMID 2030669.
- Faergeman N. J, Knudsen J. (1997). "Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling". Biochem J. 323: 1–12. PMID 9173866.
{{cite journal}}: Unknown parameter|month=ignored (help) - Doble B. W., Woodgett J. R. (2003). "GSK-3: tricks of the trade for a multi-tasking kinase". J. Cell. Sci. 116: 1175–1186. PMID 12615961.
{{cite journal}}: Unknown parameter|month=ignored (help) - Carr C. M. , Kim P. S. (2003). "A spring-loaded mechanism for the conformational change of influenza hemagglutinin". Cell. 73: 823–832. PMID 8500173.
{{cite journal}}: Unknown parameter|month=ignored (help) - Phenylketonuria: NCBI Genes and Disease
- Renugopalakrishnan V, Garduno-Juarez R, Narasimhan G, Verma CS, Wei X, Li P. (2005). "Rational design of thermally stable proteins: relevance to bionanotechnology". J Nanosci Nanotechnol. 5 (11): 1759–1767. PMID 16433409.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hult K, Berglund P. (2003). "Engineered enzymes for improved organic synthesis". Curr Opin Biotechnol. 14 (4): 395–400. PMID 12943848.
Further reading
|
Etymology and history
Enzyme structure and mechanism
Thermodynamics
|
Kinetics and inhibition
Function and control of enzymes in the cell
Enzyme-naming conventions
Industrial applications
|
External links
- Structure/Function of Enzymes, Web tutorial on enzyme structure and function.
- Enzyme Action (Flash Animation)
- Enzyme spotlight Monthly feature at the European Bioinformatics Institute on a selected enzyme.
- UK biotech and pharmaceutical industry The Biosystems Informatics Institute (Bii) is a new UK government initiative funded by the Department of Trade and Industry and the Regional Development Agency, One NorthEast. From its outset the Institute will undertake industry-facing research and development in collaboration with the UK biotech and pharmaceutical industry.
- AMFEP, Association of Manufacturers and Formulators of Enzyme Products
- ExPASy enzyme database, links to Swiss-Prot sequence data, entries in other databases and to related literature searches.
- Enzyme Structures Database links to the known 3-D structure data of enzymes in the Protein Data Bank.
- MACiE, database of enzyme reaction mechanisms.
- BRENDA, comprehensive compilation of information and literature references about all known enzymes; requires payment by commercial users.
- KEGG: Kyoto Encyclopedia of Genes and Genomes Graphical and hypertext-based information on biochemical pathways and enzymes.
- 'Face-to-Face Interview with Sir John Cornforth who was awarded a Nobel Prize for work on stereochemistry of enzyme-catalyzed reactions Freeview video by the Vega Science Trust
- How Enzymes Work Brillant Flash Animation
Template:BranchesofFoodChemistry
Template:Link FA Template:Link FA
Categories: (in
(in  (in
(in