| Kaolinite | |
|---|---|
 | |
| General | |
| Category | Phyllosilicates Kaolinite-serpentine group |
| Formula (repeating unit) | Al2Si2O5(OH)4, or in oxide notation: Al2O3·2SiO2·2H2O |
| IMA symbol | Kln |
| Strunz classification | 9.ED.05 |
| Crystal system | Triclinic |
| Crystal class | Pedial (1) (same H-M symbol) |
| Space group | P1 |
| Unit cell | a = 5.13 Å, b = 8.89 Å c = 7.25 Å; α = 90° β = 104.5°, γ = 89.8°; Z = 2 |
| Identification | |
| Color | White to cream, sometimes red, blue or brown tints from impurities and pale-yellow; also often stained various hues, tans and browns being common. |
| Crystal habit | Rarely as crystals, thin plates or stacked. More commonly as microscopic pseudohexagonal plates and clusters of plates, aggregated into compact, claylike masses. |
| Cleavage | Perfect on {001} |
| Tenacity | Flexible but inelastic |
| Mohs scale hardness | 2–2.5 |
| Luster | Pearly to dull earthy |
| Streak | White |
| Specific gravity | 2.16–2.68 |
| Optical properties | Biaxial (–) |
| Refractive index | nα = 1.553–1.565, nβ = 1.559–1.569, nγ = 1.569–1.570 |
| 2V angle | Measured: 24° to 50°, Calculated: 44° |
| References | |
Kaolinite (/ˈkeɪ.ələˌnaɪt, -lɪ-/ KAY-ə-lə-nyte, -lih-; also called kaolin) is a clay mineral, with the chemical composition Al2Si2O5(OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica (SiO4) linked through oxygen atoms to one octahedral sheet of alumina (AlO6).
Kaolinite is a soft, earthy, usually white, mineral (dioctahedral phyllosilicate clay), produced by the chemical weathering of aluminium silicate minerals like feldspar. It has a low shrink–swell capacity and a low cation-exchange capacity (1–15 meq/100 g).
Rocks that are rich in kaolinite, and halloysite, are known as kaolin (/ˈkeɪ.əlɪn/) or china clay. In many parts of the world kaolin is colored pink-orange-red by iron oxide, giving it a distinct rust hue. Lower concentrations of iron oxide yield the white, yellow, or light orange colors of kaolin. Alternating lighter and darker layers are sometimes found, as at Providence Canyon State Park in Georgia, United States.
Kaolin is an important raw material in many industries and applications. Commercial grades of kaolin are supplied and transported as powder, lumps, semi-dried noodle or slurry. Global production of kaolin in 2021 was estimated to be 45 million tonnes, with a total market value of US $4.24 billion.
Names
The English name kaolin was borrowed in 1727 from François Xavier d'Entrecolles's 1712 French reports on the manufacture of Jingdezhen porcelain. D'Entrecolles was transcribing the Chinese term 高嶺土, now romanized as gāolǐngtǔ in pinyin, taken from the name of the village of Gaoling ("High Ridge") near Ehu in Fuliang County, now part of Jiangxi Province's Jingdezhen Prefecture. The area around the village had become the main source of Jingdezhen's kaolin over the course of the Qing dynasty. The mineralogical suffix -ite was later added to generalize the name to cover nearly identical minerals from other locations.
Kaolinite is also occasionally discussed under the archaic names lithomarge and lithomarga from Latin lithomarga, a combination of litho- (Ancient Greek: λίθος,líthos, "stone") and marga ("marl"). In more proper modern use, lithomarge now refers specifically to a compacted and massive form of kaolin.
Chemistry
Notation
The chemical formula for kaolinite as written in mineralogy is Al2Si2O5(OH)4, however, in ceramics applications the same formula is typically written in terms of oxides, thus giving Al2O3·2SiO2·2H2O.
Structure

Compared with other clay minerals, kaolinite is chemically and structurally simple. It is described as a 1:1 or TO clay mineral because its crystals consist of stacked TO layers. Each TO layer consists of a tetrahedral (T) sheet composed of silicon and oxygen ions bonded to an octahedral (O) sheet composed of oxygen, aluminium, and hydroxyl ions. The T sheet is so called because each silicon ion is surrounded by four oxygen ions forming a tetrahedron. The O sheet is so called because each aluminium ion is surrounded by six oxygen or hydroxyl ions arranged at the corners of an octahedron. The two sheets in each layer are strongly bonded together via shared oxygen ions, while layers are bonded via hydrogen bonding between oxygen on the outer face of the T sheet of one layer and hydroxyl on the outer face of the O sheet of the next layer.
-
 View of the structure of the tetrahedral (T) sheet of kaolinite
View of the structure of the tetrahedral (T) sheet of kaolinite
-
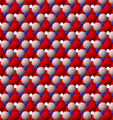 View of the structure of the octahedral (O) sheet of kaolinite
View of the structure of the octahedral (O) sheet of kaolinite
-
 Kaolinite crystal structure looking along the layers
Kaolinite crystal structure looking along the layers
A kaolinite layer has no net electrical charge and so there are no large cations (such as calcium, sodium, or potassium) between layers as with most other clay minerals. This accounts for kaolinite's relatively low ion exchange capacity. The close hydrogen bonding between layers also hinders water molecules from infiltrating between layers, accounting for kaolinite's nonswelling character.
When moistened, the tiny platelike crystals of kaolinite acquire a layer of water molecules that cause crystals to adhere to each other and give kaolin clay its cohesiveness. The bonds are weak enough to allow the plates to slip past each other when the clay is being molded, but strong enough to hold the plates in place and allow the molded clay to retain its shape. When the clay is dried, most of the water molecules are removed, and the plates hydrogen bond directly to each other, so that the dried clay is rigid but still fragile. If the clay is moistened again, it will once more become plastic.
Structural transformations
Kaolinite group clays undergo a series of phase transformations upon thermal treatment in air at atmospheric pressure.
Milling
High-energy milling of kaolin results in the formation of a mechanochemically amorphized phase similar to metakaolin, although the properties of this solid are quite different. The high-energy milling process is highly inefficient and consumes a large amount of energy.
Drying
See also: Buell dryerBelow 100 °C, exposure to low humidity air will result in the slow evaporation of any liquid water in the kaolin. At low moisture content the mass can be described leather dry, and at near 0% moisture it is referred to as bone dry.
Above 100 °C any remaining free water is lost. Above around 400 °C hydroxyl ions (OH) are lost from the kaolinite crystal structure in the form of water: the material cannot now be plasticised by absorbing water. This is irreversible, as are subsequent transformations; this is referred to as calcination.
Metakaolin
Endothermic dehydration of kaolinite begins at 550–600 °C producing disordered metakaolin, but continuous hydroxyl loss is observed up to 900 °C (1,650 °F). Although historically there was much disagreement concerning the nature of the metakaolin phase, extensive research has led to a general consensus that metakaolin is not a simple mixture of amorphous silica (SiO2) and alumina (Al2O3), but rather a complex amorphous structure that retains some longer-range order (but not strictly crystalline) due to stacking of its hexagonal layers.
Spinel
Further heating to 925–950 °C converts metakaolin to an aluminium-silicon spinel which is sometimes also referred to as a gamma-alumina type structure:
Platelet mullite
Upon calcination above 1050 °C, the spinel phase nucleates and transforms to platelet mullite and highly crystalline cristobalite:
Needle mullite
Finally, at 1400 °C the "needle" form of mullite appears, offering substantial increases in structural strength and heat resistance. This is a structural but not chemical transformation. See stoneware for more information on this form.
Occurrence

Kaolinite is one of the most common minerals; it is mined, as kaolin, in Australia, Brazil, Bulgaria, China, Czech Republic, France, Germany, India, Iran, Malaysia, South Africa, South Korea, Spain, Tanzania, Thailand, United Kingdom, United States and Vietnam.
Mantles of kaolinite are common in Western and Northern Europe. The ages of these mantles are Mesozoic to Early Cenozoic.
Kaolinite clay occurs in abundance in soils that have formed from the chemical weathering of rocks in hot, moist climates; for example in tropical rainforest areas. Comparing soils along a gradient towards progressively cooler or drier climates, the proportion of kaolinite decreases, while the proportion of other clay minerals such as illite (in cooler climates) or smectite (in drier climates) increases. Such climatically related differences in clay mineral content are often used to infer changes in climates in the geological past, where ancient soils have been buried and preserved.

In the Institut National pour l'Étude Agronomique au Congo Belge (INEAC) classification system, soils in which the clay fraction is predominantly kaolinite are called kaolisol (from kaolin and soil).
In the United States, the main kaolin deposits are found in central Georgia, on a stretch of the Atlantic Seaboard fall line between Augusta and Macon. This area of thirteen counties is called the "white gold" belt; Sandersville is known as the "Kaolin Capital of the World" due to its abundance of kaolin. In the late 1800s, an active kaolin surface-mining industry existed in the extreme southeast corner of Pennsylvania, near the towns of Landenberg and Kaolin, and in what is present-day White Clay Creek Preserve. The product was brought by train to Newark, Delaware, on the Newark-Pomeroy line, along which can still be seen many open-pit clay mines. The deposits were formed between the late Cretaceous and early Paleogene, about 100 to 45 million years ago, in sediments derived from weathered igneous and metakaolin rocks. Kaolin production in the United States during 2011 was 5.5 million tons.

During the Paleocene–Eocene Thermal Maximum sediments deposited in the Espluga Freda area of Spain were enriched with kaolinite from a detrital source due to denudation.
Synthesis and genesis
Difficulties are encountered when trying to explain kaolinite formation under atmospheric conditions by extrapolation of thermodynamic data from the more successful high-temperature syntheses. La Iglesia and Van Oosterwijk-Gastuche (1978) thought that the conditions under which kaolinite will nucleate can be deduced from stability diagrams, based as they are on dissolution data. Because of a lack of convincing results in their own experiments, La Iglesia and Van Oosterwijk-Gastuche (1978) had to conclude, however, that there were other, still unknown, factors involved in the low-temperature nucleation of kaolinite. Because of the observed very slow crystallization rates of kaolinite from solution at room temperature Fripiat and Herbillon (1971) postulated the existence of high activation energies in the low-temperature nucleation of kaolinite.
At high temperatures, equilibrium thermodynamic models appear to be satisfactory for the description of kaolinite dissolution and nucleation, because the thermal energy suffices to overcome the energy barriers involved in the nucleation process. The importance of syntheses at ambient temperature and atmospheric pressure towards the understanding of the mechanism involved in the nucleation of clay minerals lies in overcoming these energy barriers. As indicated by Caillère and Hénin (1960) the processes involved will have to be studied in well-defined experiments, because it is virtually impossible to isolate the factors involved by mere deduction from complex natural physico-chemical systems such as the soil environment. Fripiat and Herbillon (1971), in a review on the formation of kaolinite, raised the fundamental question how a disordered material (i.e., the amorphous fraction of tropical soils) could ever be transformed into a corresponding ordered structure. This transformation seems to take place in soils without major changes in the environment, in a relatively short period of time, and at ambient temperature (and pressure).
Low-temperature synthesis of clay minerals (with kaolinite as an example) has several aspects. In the first place the silicic acid to be supplied to the growing crystal must be in a monomeric form, i.e., silica should be present in very dilute solution (Caillère et al., 1957; Caillère and Hénin, 1960; Wey and Siffert, 1962; Millot, 1970). In order to prevent the formation of amorphous silica gels precipitating from supersaturated solutions without reacting with the aluminium or magnesium cations to form crystalline silicates, the silicic acid must be present in concentrations below the maximum solubility of amorphous silica. The principle behind this prerequisite can be found in structural chemistry: "Since the polysilicate ions are not of uniform size, they cannot arrange themselves along with the metal ions into a regular crystal lattice." (Iler, 1955, p. 182)
The second aspect of the low-temperature synthesis of kaolinite is that the aluminium cations must be hexacoordinated with respect to oxygen (Caillère and Hénin, 1947; Caillère et al., 1953; Hénin and Robichet, 1955). Gastuche et al. (1962) and Caillère and Hénin (1962) have concluded that kaolinite can only ever be formed when the aluminium hydroxide is in the form of gibbsite. Otherwise, the precipitate formed will be a "mixed alumino-silicic gel" (as Millot, 1970, p. 343 put it). If it were the only requirement, large amounts of kaolinite could be harvested simply by adding gibbsite powder to a silica solution. Undoubtedly a marked degree of adsorption of the silica in solution by the gibbsite surfaces will take place, but, as stated before, mere adsorption does not create the layer lattice typical of kaolinite crystals.
The third aspect is that these two initial components must be incorporated into one mixed crystal with a layer structure. From the following equation (as given by Gastuche and DeKimpe, 1962) for kaolinite formation
it can be seen that five molecules of water must be removed from the reaction for every molecule of kaolinite formed. Field evidence illustrating the importance of the removal of water from the kaolinite reaction has been supplied by Gastuche and DeKimpe (1962). While studying soil formation on a basaltic rock in Kivu (Zaïre), they noted how the occurrence of kaolinite depended on the "degrée de drainage" of the area involved. A clear distinction was found between areas with good drainage (i.e., areas with a marked difference between wet and dry seasons) and those areas with poor drainage (i.e., perennially swampy areas). Kaolinite was only found in the areas with distinct seasonal alternations between wet and dry. The possible significance of alternating wet and dry conditions on the transition of allophane into kaolinite has been stressed by Tamura and Jackson (1953). The role of alternations between wetting and drying on the formation of kaolinite has also been noted by Moore (1964).
Laboratory syntheses
Syntheses of kaolinite at high temperatures (more than 100 °C ) are relatively well known. There are for example the syntheses of Van Nieuwenberg and Pieters (1929); Noll (1934); Noll (1936); Norton (1939); Roy and Osborn (1954); Roy (1961); Hawkins and Roy (1962); Tomura et al. (1985); Satokawa et al. (1994) and Huertas et al. (1999). Relatively few low-temperature syntheses have become known (cf. Brindley and DeKimpe (1961); DeKimpe (1969); Bogatyrev et al. (1997)).
Laboratory syntheses of kaolinite at room temperature and atmospheric pressure have been described by DeKimpe et al. (1961). From those tests the role of periodicity becomes convincingly clear. DeKimpe et al. (1961) had used daily additions of alumina (as AlCl3·6 H2O) and silica (in the form of ethyl silicate) during at least two months. In addition, adjustments of the pH took place every day by way of adding either hydrochloric acid or sodium hydroxide. Such daily additions of Si and Al to the solution in combination with the daily titrations with hydrochloric acid or sodium hydroxide during at least 60 days will have introduced the necessary element of periodicity. Only now the actual role of what has been described as the "aging" (Alterung) of amorphous alumino-silicates (as for example Harder, 1978 had noted) can be fully understood. As such, time is not bringing about any change in a closed system at equilibrium; but a series of alternations of periodically changing conditions (by definition, taking place in an open system) will bring about the low-temperature formation of more and more of the stable phase kaolinite instead of (ill-defined) amorphous alumino-silicates.
Applications
Main
In 2009, up to 70% of kaolin was used in the production of paper. Following reduced demand from the paper industry, resulting from both competing minerals and the effect of digital media, in 2016 the market share was reported to be: paper, 36%; ceramics, 31%; paint, 7% and other, 26%. According to the USGS, in 2021 the global production of kaolin was estimated to be around 45 million tonnes.
- Paper applications require high-brightness, low abrasion and delaminated kaolins. For paper coatings it is used to enhance the gloss, brilliance, smoothness and receptability to inks; it can account for 25% of mass of the paper. As a paper filler it is used as a pulp extender, and to increase opacity; it can account for 15% of mass.
- In whiteware ceramic bodies, kaolin can constitute up to 50% of the raw materials. In unfired bodies it contributes to the green strength, plasticity and rheological properties, such as the casting rate. During firing it reacts with other body components to form the crystal and glass phases. With suitable firing schedules it is key to the formation of mullite. The most valued grades have low contents of chromophoric oxides such that the fired material has high whiteness. In glazes it is primarily used as a rheology control agent, but also contributes some green strength. In both glazes and frits it contributes some SiO2 as a glass network former, and Al2O3 as both a network former and modifier.
Other industrial
- As a raw material for the production of an insulation material called Kaowool (a form of mineral wool).
- An additive to some paints to extend the titanium dioxide (TiO2) white pigment and modify gloss levels.
- An additive to modify the properties of rubber upon vulcanization.
- An additive to adhesives to modify rheology.
- As adsorbents in water and wastewater treatment.
- In its altered metakaolin form, as a pozzolan; when added to a concrete mix, metakaolin accelerates the hydration of Portland cement and takes part in the pozzolanic reaction with the portlandite formed in the hydration of the main cement minerals (e.g. alite).
- Metakaolin is also a base component for geopolymer compounds.
Medical
- To soothe an upset stomach, similar to the way parrots (and later, humans) in South America originally used it (more recently, industrially produced).
- Kaolin-based preparations are used for treatment of diarrhea.
- An ingredient in 'pre-work' skin protection and barrier creams.
- To induce and accelerate blood clotting. In April 2008 the US Naval Medical Research Institute announced the successful use of a kaolinite-derived aluminosilicate infusion in traditional gauze. which is still the hemostat of choice for all branches of the US military. See Kaolin clotting time and quikclot
- As a mild abrasive in toothpaste.
Cosmetics
- As a filler in cosmetics.
- For facial masks or soap.
- for spa body treatments, such as body wraps, cocoons, or spot treatments.
Archaeology
- As an indicator in radiological dating since kaolinite can contain very small traces of uranium and thorium.
Geophagy
- Humans sometimes eat kaolin for pleasure or to suppress hunger, a practice known as geophagy. In Africa, kaolin used for such purposes is known as kalaba (in Gabon and Cameroon), calaba, and calabachop (in Equatorial Guinea). Consumption is greater among women, especially during pregnancy, and its use is sometimes said by women of the region to be a habit analogous to cigarette smoking among men. The practice has also been observed within a small population of African-American women in the Southern United States, especially Georgia, likely brought with the traditions of the aforementioned Africans via slavery. There, the kaolin is called white dirt, chalk or white clay.
Geotechnical engineering
- Research results show that the utilization of kaolinite in geotechnical engineering can be alternatively replaced by safer illite, especially if its presence is less than 10.8% of the total rock mass.
Small-scale uses
- As a light-diffusing material in white incandescent light bulbs.
- In organic farming as a spray applied to crops to deter insect damage, and in the case of apples, to prevent sun scald.
- As whitewash in traditional stone masonry homes in Nepal.
- As a filler in Edison Diamond Discs.
Production output
Global production of kaolin by country in 2012 was estimated to be:
| Global - total | 26,651 |
|---|---|
| Egypt | 275 |
| Nigeria | 100 |
| Algeria | 80 |
| Tanzania | 45 |
| Sudan | 35 |
| Uganda | 30 |
| South Africa | 15 |
| Ethiopia | 2 |
| Kenya | 1 |
| Africa - total | 583 |
| China | 3,950 |
| South Korea | 800 |
| Vietnam | 650 |
| Malaysia | 450 |
| Thailand | 180 |
| Indonesia' | 175 |
| India | 75 |
| Bangladesh | 20 |
| Taiwan | 17 |
| Pakistan | 15 |
| Sri Lanka | 11 |
| Japan | 3 |
| Philippines | 2 |
| Asia - total | 6,348 |
| Germany | 4,800 |
| UK | 1,000 |
| Czech Republic | 650 |
| Italy | 625 |
| France | 350 |
| Portugal | 325 |
| Spain | 300 |
| Bosnia–Herzegovina | 250 |
| Bulgaria | 225 |
| Russia | 170 |
| Poland | 125 |
| Ukraine | 100 |
| Serbia | 90 |
| Austria | 65 |
| Denmark | 3 |
| Europe - total | 9,078 |
| USA | 5,900 |
| Mexico | 120 |
| N. America - total | 6,020 |
| Iran | 1,500 |
| Turkey | 725 |
| Jordan | 100 |
| Saudi Arabia | 70 |
| Iraq | 3 |
| Middle East - total | 2,398 |
| Australia | 40 |
| New Zealand | 11 |
| Oceania - total | 51 |
| Brazil | 1,900 |
| Argentina | 80 |
| Paraguay | 66 |
| Chile | 60 |
| Colombia | 20 |
| Peru | 20 |
| Ecuador | 15 |
| Venezuela | 10 |
| Guatemala | 2 |
| S. & C. America - total | 2,173 |
Typical properties
Some selected typical properties of various ceramic grade kaolins are:
| Product name | SSP | Premium | Longyan 325# | Zettlitz 1A | OKA |
|---|---|---|---|---|---|
| Country | UK | New Zealand | China | Czech Republic | Germany |
| Manufacturer | Imerys | Imerys | Logyan | Sedlecky | AKW |
| % < 2 μm | 85 | 97 | 25 | 56 | 82 |
| % <1 μm | 50 | 88 | 15 | 41 | 50 |
| SiO2, % | 48.0 | 49.5 | 49.3 | 48.0 | 49.5 |
| Al2O3, % | 37.0 | 35.5 | 35.5 | 37.0 | 35.5 |
| Fe2O3, % | 0.44 | 0.29 | 0.22 | 0.68 | 0.43 |
| TiO2, % | 0.01 | 0.09 | 0.01 | 0.20 | 0.17 |
| CaO, % | 0.10 | - | 0.03 | 0.08 | 0.20 |
| MgO, % | 0.25 | - | 0.25 | 0.23 | 0.02 |
| K2O, % | 1.25 | - | 1.90 | 0.92 | 0.30 |
| Na2O, % | 0.15 | - | 0.09 | 0.07 | 0.01 |
| LOI, % | 12.8 | 13.8 | 11.9 | 12.9 | 13.4 |
| Kaolinite, % | 95 | - | 40 | 89 | 86 |
| Halloysite, % | - | 92 | 40 | - | - |
| Mica, % | 4 | - | - | - | - |
| Quartz, % | 1 | 4 | 3 | 1 | 8 |
| Smectite, % | - | - | - | 1 | 6 |
| Cristobalite, % | - | 4 | - | - | - |
Safety
| NFPA 704 safety square | |
|---|---|
 |
Kaolin is generally recognized as safe, but may cause mild irritation of the skin or mucous membranes. Kaolin products may also contain traces of crystalline silica, a known carcinogen if inhaled.
In the US, the Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for kaolin exposure in the workplace as 15 mg/m total exposure and 5 mg/m respiratory exposure over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 10 mg/m total exposure TWA 5 mg/m respiratory exposure over an 8-hour workday.
See also
- China stone – Type of altered granite
- Clay pit – Open-pit mining for the extraction of clay minerals
- Dickite – Phyllosilicate mineral
- Halloysite – Aluminosilicate clay mineral
- Kaolin Deposits of Charentes Basin, France – Sedimentary clay deposits in FrancePages displaying short descriptions of redirect targets
- Kaolin spray – Kaolin-based pest control
- Medicinal clay – Use of clay for health reasons
- Nacrite – Phyllosilicate mineral: group of kaolinite
- Cornish China Clay Branches – Railway branch lines in England
References
Citations
- Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ "Kaolinite". Mindat.org. Retrieved 5 August 2009.
- Kaolinite Mineral Data, WebMineral.com, retrieved 5 August 2009
- ^ Anthony JW, Bideaux RA, Bladh KW, et al., eds. (1995). "Kaolinite" (PDF). Handbook of Mineralogy: Silica, silicates. Tucson, Ariz.: Mineral Data Publishing. ISBN 9780962209734. OCLC 928816381.
- "kaolinite". Dictionary.com Unabridged (Online). n.d.
- "kaolinite". Lexico UK English Dictionary. Oxford University Press. Archived from the original on 25 January 2021.
- "kaolinite". The American Heritage Dictionary of the English Language (5th ed.). HarperCollins.
- Deer, Howie & Zussman (1992).
- Pohl WL (2011). Economic geology: principles and practice: metals, minerals, coal and hydrocarbons – introduction to formation and sustainable exploitation of mineral deposits. Chichester, West Sussex: Wiley-Blackwell. p. 331. ISBN 9781444336627.
- 'U.S. Geological Survey, Mineral Commodity Summaries, January 2022' USGS, 2022.
- 'Kaolin Market Size, Share & Trends Analysis Report By Application, By Region And Segment Forecasts, 2022 - 2030. Grand View Research, 2022
- Harper, Douglas. "kaolin". Online Etymology Dictionary.
- ^ Schroeder PA (31 July 2018). "Kaolin". New Georgia Encyclopedia (online). Retrieved 14 March 2019.
- ^ Needham & al. (2004), p. 220.
- "Lithomarge". www.mindat.org. Retrieved 23 February 2022.
- Perry DL (2011). Handbook of Inorganic Compounds (2nd ed.). Boca Raton: Taylor & Francis. ISBN 9781439814611. OCLC 587104373.
- ^ Nesse, William D. (2000). Introduction to mineralogy. New York: Oxford University Press. pp. 254–255. ISBN 9780195106916.
- Breuer, Stephen (July 2012). "The chemistry of pottery" (PDF). Education in Chemistry: 17–20. Retrieved 8 December 2020.
- Kasa E, Szabados M, Baan K, Konya Z, Kukovecz A, Kutus B, Palinko I, Sipos P (2021). "The dissolution kinetics of raw and mechanochemically treated kaolinites in industrial spent liquor – The effect of the physico-chemical properties of the solids". Appl. Clay Sci. 203: 105994. Bibcode:2021ApCS..20305994K. doi:10.1016/j.clay.2021.105994. hdl:21.11116/0000-0008-06AA-2.
- Baláž, Peter (2008). "High-Energy Milling". Mechanochemistry in Nanoscience and Minerals Engineering. pp. 103–132. doi:10.1007/978-3-540-74855-7_2. ISBN 978-3-540-74854-0.
- 'Ceramics Are More Than Clay Alone - Raw Materials, Products, Applications' P. Bormans. Cambridge International Science Publishing, 2004. pg. 180
- ^ Bellotto M, Gualtieri A, Artioli G, et al. (1995). "Kinetic study of the kaolinite-mullite reaction sequence. Part I: kaolinite dehydroxylation". Phys. Chem. Miner. 22 (4): 207–214. Bibcode:1995PCM....22..207B. doi:10.1007/BF00202253. S2CID 95897543.
- Migoń P, Lidmar-Bergström K (2002). "Deep weathering through time in central and northwestern Europe: problems of dating and interpretation of geological record". Catena. 49 (1–2): 25–40. Bibcode:2002Caten..49...25M. doi:10.1016/S0341-8162(02)00015-2.
- Girard, Jean-Pierre; Freyssinet, Philippe; Chazot, Gilles (1 February 2000). "Unraveling climatic changes from intraprofile variation in oxygen and hydrogen isotopic composition of goethite and kaolinite in laterites: an integrated study from Yaou, French Guiana". Geochimica et Cosmochimica Acta. 64 (3): 409–426. Bibcode:2000GeCoA..64..409G. doi:10.1016/S0016-7037(99)00299-9. ISSN 0016-7037.
- Young A (1980). Tropical soils and soil survey. Cambridge Geographical Studies. Vol. 9. CUP Archive. p. 132. ISBN 9780521297684.
- "Kaolin Capital of the World". City of Sandersville, GA. Retrieved 27 August 2018.
- Reece C. "Making Peace With the Age-Old Practice of Eating White Dirt". The Bitter Southerner. Retrieved 27 August 2018.
- Smothers, Ronald (12 December 1987). "White George clay turns into cash". The New York Times. Retrieved 19 January 2021.
- Virta R (2012). Mineral Commodity Summaries (PDF) (Technical report). U.S. Geological Survey. pp. 44–45.
- Adatte T, Khozyem H, Spangenberg JE, et al. (2014). "Response of terrestrial environment to the Paleocene-Eocene Thermal Maximum (PETM), new insights from India and NE Spain". Rendiconti Online della Società Geologica Italiana. 31: 5–6. doi:10.3301/ROL.2014.17.
- Meijer EL, van der Plas L (1980). Relative stabilities of soil minerals. Mededelingen Landbouwhogeschool Wageningen. Vol. 80. Wageningen: Veenman. p. 18.
- La Iglesia A, Van Oosterwyck-Gastuche MC (1978). "Kaolinite Synthesis. I. Crystallization Conditions at Low Temperatures and Calculation of Thermodynamic Equilibria. Application to Laboratory and Field Observations". Clays and Clay Minerals. 26 (6): 397–408. Bibcode:1978CCM....26..397L. doi:10.1346/CCMN.1978.0260603.
- ^ Caillère S, Hénin S (1960). "Vues d'ensemble sur le problème de la synthèse des minéraux argileux à basse température". Bulletin du Groupe français des argiles (in French). 12 (7): 63. doi:10.3406/argil.1960.969.
- Fripiat JJ, Herbillon AJ (1971). "Formation and transformations of clay minerals in tropical soils". Soils and tropical weathering: proceedings of the Bandung Symposium 16 to 23 November 1969. Natural resources research. Vol. 11. Paris: Unesco. pp. 15–24. OCLC 421565.
- Caillère S, Hénin S, Esquevin J (1957). "Synthèse des minéraux argileux". Bulletin du Groupe français des argiles (in French). 9 (4): 67–76. doi:10.3406/argil.1957.940.
- Wey R, Siffert B (1961). "Réactions de la silice monomoléculaire en solutions avec les ions Al3+ et Mg2+". Colloques Internationaux (in French). 105. Centre National des Recherches Scientifiques: 11–23.
- Millot G (1970). Geology of Clays. Translated by Paquet H, Farrand WR. New York: Springer-Verlag. doi:10.1007/978-3-662-41609-9. ISBN 9783662416099. S2CID 128831318.
- Iler RK (1955). The colloid chemistry of silica and silicates. Ithaca, N.Y.: Cornell University Press.
- Caillère S, Hénin S (1947). "Formation d'une phyllite du type kaolinique par traitement d'une montmorillonite". Comptes Rendus de l'Académie des Sciences de Paris. 224 (1): 53–55.
- Caillère S, Hénin S, Esquevin J (1953). "Recherches sur la synthèse des minéraux argileux". Bulletin de la Société française de Minéralogie et de Cristallographie (in French). 76 (7): 300–314. doi:10.3406/bulmi.1953.4841.
- Hénin S, Robichet O (1955). "Résultats obtenus au cours de nouveaux essais de synthèse de minéraux argileux". Bulletin du Groupe français des argiles (in French). 6 (1): 19–22. doi:10.3406/argil.1955.1257.
- Gastuche MC, Fripiat JJ, DeKimpe C (1962). "La genèse des minéraux argileux de la famille du kaolin. I. – Aspect colloidal". Colloque C.N.R.S. 105: 57–65.
- Gastuche MC, DeKimpe C (1962). "La genèse des minéraux argileux de la famille du kaolin. II. Aspect cristallin". Colloque C.N.R.S. 105: 75–88.
- Tamura T, Jackson ML (1953). "Structural and Energy Relationships in the Formation of Iron and Aluminum Oxides, Hydroxides, and Silicates". Science. 117 (3041): 381–383. Bibcode:1953Sci...117..381T. doi:10.1126/science.117.3041.381. PMID 17749950.
- Moore LR (1964). "The in Situ Formation and Development of Some Kaolinite Macrocrystals". Clay Minerals. 5 (31): 338–352. Bibcode:1964ClMin...5..338M. doi:10.1180/claymin.1964.005.31.02.
- van Nieuwenburg CJ, Pieters HA (1929). "Studies on hydrated aluminium silicates: I. The rehydration of metakaolin and the synthesis of kaolin". Recl. Trav. Chim. Pays-Bas. 48 (1): 27–36. doi:10.1002/recl.19290480106.
- Noll W (1934). "Hydrothermale Synthese des Kaolins". Zeitschrift für Kristallographie, Mineralogie und Petrographie (in German). 45 (2–3): 175–190. Bibcode:1934ZKMP...45..175N. doi:10.1007/BF02943371. S2CID 96869398.
- Noll W (1936). "Über die Bildungsbedingungen von Kaolin, Montmorillonit, Sericit, Pyrophyllit und Analcim". Zeitschrift für Kristallographie, Mineralogie und Petrographie (in German). 48 (3–4): 210–247. Bibcode:1936ZKMP...48..210N. doi:10.1007/BF02939458. S2CID 128744123.
- Norton FH (1939). "Hydrothermal formation of clay minerals in the laboratory". Am. Mineral. 24 (1): 1–17.
- Roy R, Osborn EF (1954). "The system Al2O3-SiO2-H2O". Am. Mineral. 39 (11–12): 853–885.
- Roy R (1962). "The preparation and properties of synthetic clay minerals". Colloque C.N.R.S. 105: 83–98.
- Hawkins DB, Roy R (1962). "Electrolytic Synthesis of Kaolinite Under Hydrothermal Conditions". J. Am. Ceram. Soc. 45 (10): 507–508. doi:10.1111/j.1151-2916.1962.tb11044.x.
- Tomura S, Shibasaki Y, Mizuta H, et al. (1985). "Growth Conditions and Genesis of Spherical and Platy Kaolinite". Clays and Clay Minerals. 33 (3): 200–206. Bibcode:1985CCM....33..200T. doi:10.1346/CCMN.1985.0330305.
- Satokawa S, Osaki Y, Samejima S, et al. (1994). "Effects of the Structure of Silica-Alumina Gel on the Hydrothermal Synthesis of Kaolinite". Clays and Clay Minerals. 42 (3): 288–297. Bibcode:1994CCM....42..288S. doi:10.1346/CCMN.1994.0420307.
- Huertas FJ, Fiore S, Huertas F, et al. (1999). "Experimental study of the hydrothermal formation of kaolinite". Chemical Geology. 156 (1–4): 171–190. Bibcode:1999ChGeo.156..171H. doi:10.1016/S0009-2541(98)00180-6.
- Brindley GW, De Kimpe C (1961). "Attempted Low-Temperature Syntheses of Kaolin Minerals". Nature. 190 (4772): 254. Bibcode:1961Natur.190..254B. doi:10.1038/190254a0. S2CID 4149442.
- De Kimpe CR (1969). "Crystallization of kaolinite at low temperature from an alumino-silicic gel". Clays and Clay Minerals. 17 (1): 37–38. Bibcode:1969CCM....17...37D. doi:10.1346/CCMN.1969.0170107.
- Bogatyrev BA, Mateeva LA, Zhukov VV, et al. (1997). "Low-temperature synthesis of kaolinite and halloysite on the gibbsite – silicic acid solution system". Transactions (Doklady) of the Russian Academy of Sciences. Earth science sections. 353 A: 403–405.
- DeKimpe CR, Gastuche MC, Brindley GW (1961). "Ionic coordination in alumino-silicic acids in relation to clay mineral formation" (PDF). Am. Mineral. 46 (11–12): 1370–1381.
- Harder H (1978). "Synthesen von Tonmineralen unter spezieller Berücksichtigung festländischer Bedingungen". Schriftenreihe für geologische Wissenschaften (Berlin) (in German). 11: 51–78.
- ^ 'Positive Outlook For Kaolin In Ceramics' F. Hart, I. Wilson. Industrial Minerals, April 2019. Pg.28
- King, R.J. (March 2009). "Kaolinite". Geology Today. 25 (2): 75–78. Bibcode:2009GeolT..25...75K. doi:10.1111/j.1365-2451.2009.00711.x. S2CID 242917623.
- U.S. Geological Survey, Mineral Commodity Summaries, January 2022
- ’Industrial Minerals And Their Uses - A Handbook And Formulary. P. A. Ciullo. William Andrew, 1996. Pg. 43
- ^ 'Kaolin - Global Markets And Industry Outlook' 13th edition. Roskill Information Services, 2013. Pg. 332
- Murray HH, Lyons SC (1955). "Correlation of Paper-Coating Quality with Degree of Crystal Perfection of Kaolinite". Clays and Clay Minerals. 4 (1): 31–40. Bibcode:1955CCM.....4...31M. doi:10.1346/CCMN.1955.0040105.
- ’Industrial Minerals And Their Uses - A Handbook And Formular’ P. A. Ciullo. William Andrew, 1996. Pg. 43
- ’Dictionary Of Ceramic Science And Engineering' L. S. O’Bannon. Plenum Press / Springer. 1984. Pg.146
- ’Dictionary Of Ceramic Science And Engineering’ 3rd edition. I. MCcolm. Springer, 2013
- 'Ceramics Glaze Technology.' J.R.Taylor & A.C.Bull. The Institute Of Ceramics/Pergamon Press986
- Ciullo PA (1996). Industrial Minerals and Their Uses: A Handbook and Formulary. Westwood, NJ: Noyes Publications. pp. 41–43. ISBN 9780815518082.
- Leiviskä T, Gehör S, Eijärvi E, et al. (2012). "Characteristics and potential applications of coarse clay fractions from Puolanka, Finland". Open Eng. 2 (2): 239–247. Bibcode:2012CEJE....2..239L. doi:10.2478/s13531-011-0067-9.
- Diamond JM (1999). "Dirty eating for healthy living". Nature. Evolutionary biology. 400 (6740): 120–121. Bibcode:1999Natur.400..120D. doi:10.1038/22014. PMID 10408435.
- "Stokoderm Protect PURE" (PDF). debgroup.com (product leaflet). Deb USA, Inc. 2017. Retrieved 12 April 2018.
- Rowe A (24 April 2008). "Nanoparticles Help Gauze Stop Gushing Wounds". Wired. Condé Nast. Archived from the original on 6 July 2009. Retrieved 5 August 2009.
- ^ Kamtche F (2012). "Balengou: autour des mines" [Balengou: around the mines]. Le Jour (in French). Archived from the original on 4 March 2012. Retrieved 22 March 2019.
- Karine Boucher, Suzanne Lafage. "Le lexique français du Gabon: K." Le Français en Afrique: Revue du Réseau des Observatoires du Français Contemporain en Afrique. 2000.
- Gerald N. Callahan. "Eating Dirt." Emerging Infectious Diseases. 9.8 (August 2003).
- ^ Grigsby RK (3 February 2004). "Clay Eating". New Georgia Encyclopedia (online). Science & Medicine. Retrieved 20 October 2019.
- Chen L (2 April 2014). "The Old And Mysterious Practice of Eating Dirt, Revealed". The Salt. NPR.
- Supandi, Supandi; Zakaria, Zufialdi; Sukiyah, Emi; Sudradjat, Adjat (29 August 2019). "The Influence of Kaolinite - Illite toward mechanical properties of Claystone". Open Geosciences. 11 (1): 440–446. Bibcode:2019OGeo...11...35S. doi:10.1515/geo-2019-0035.
- Gracyk T (2006). "Edison Diamond Discs: 1912 - 1929". Tim Gracyk's Phonographs, Singers, & Old Records. Retrieved 22 March 2019.
- 'Kaolin - Global Markets And Industry Outlook' 13th edition. Roskill Information Services, 2013. Pgs. 28-30
- ^ "Material Safety Data Sheet: Kaolin" (PDF). Connecticut College. Imerys Pigments and Additives Group. Retrieved 11 November 2021.
- "Kaolin". NIOSH Pocket Guide to Chemical Hazards. CDC. Retrieved 6 November 2015.
General references
- Breck, D. W. (1984). Zeolite Molecular Sieves. Malabar: R.E. Krieger Publishing Co. pp. 314–315. ISBN 0898746485.
- Deer, W. A.; Howie, R.A.; Zussman, J. (1992). An Introduction to the Rock-Forming Minerals (2nd ed.). Harlow: Longman. ISBN 0582300940.
- Hurlbut, C. S.; et al. (1985). Manual of Mineralogy... (20th ed.). Wiley. pp. 428–429. ISBN 0471805807.
- Needham, Joseph; et al. (2004), "Chinese Porcelain and the City of Ching-te-chen", Science and Civilisation in China, Vol. 5: Chemistry and Chemical Technology, Part XII: Ceramic Technology, Cambridge: Cambridge University Press, pp. 184–240, ISBN 0-521-83833-9.
- Schroeder, Paul A.; et al., eds. (June 2014). "Kaolin" (PDF). Elements. 10 (3). Retrieved 14 September 2022.
External links
| Clay minerals | |
|---|---|



