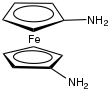
| |
| Names | |
|---|---|
| IUPAC name 1,1'-Diaminoferrocene | |
| Systematic IUPAC name Ferrocene-1,1'-diamine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H12FeN2 |
| Molar mass | 216.065 g·mol |
| Appearance | yellow solid |
| Density | 1.644 g/cm |
| Melting point | 183–186 °C (361–367 °F; 456–459 K) |
| Boiling point | decomposition |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
1,1'-Diaminoferrocene is the organoiron compound with the formula Fe(C5H4NH2)2. It is the simplest diamine derivative of ferrocene. It is a yellow, air-sensitive solid that is soluble in aqueous acid. The 1,1' part of its name refers to the location of the amine groups on separate rings. Compared to the parent ferrocene, the diamine is about 600 mV more reducing.
It can be prepared from the diisocyanate Fe(C5H4NCO)2, which in turn is derived from 1,1'-ferrocenedicarboxylic acid. 1,1'-Diaminoferrocene was originally prepared by hydrogenation of 1,1'-diazidoferrocene ( Fe(C5H4N3)2).
1,1'-Diaminoferrocene has been incorporated into various diamide and diimine ligands, which form catalysts that exhibit redox switching.
References
- ^ Shafir, Alexandr; Power, Maurice P.; Whitener, Glenn D.; Arnold, John (2000). "Synthesis, Structure, and Properties of 1,1'-Diamino- and 1,1'-Diazidoferrocene". Organometallics. 19 (19): 3978–3982. doi:10.1021/om0004085.
- Petrov, Alex R.; Jess, Kristof; Freytag, Matthias; Jones, Peter G.; Tamm, Matthias (2013). "Large-Scale Preparation of 1,1′-Ferrocenedicarboxylic Acid, a Key Compound for the Synthesis of 1,1′-Disubstituted Ferrocene Derivatives". Organometallics. 32 (20): 5946–5954. doi:10.1021/om4004972.
- Wang, Xinke; Thevenon, Arnaud; Brosmer, Jonathan L.; Yu, Insun; Khan, Saeed I.; Mehrkhodavandi, Parisa; Diaconescu, Paula L. (2014). "Redox Control of Group 4 Metal Ring-Opening Polymerization Activity toward l-Lactide and ε-Caprolactone". Journal of the American Chemical Society. 136 (32): 11264–11267. Bibcode:2014JAChS.13611264W. doi:10.1021/ja505883u. PMID 25062499. S2CID 22098566.