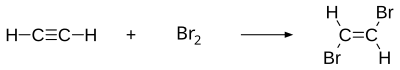| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,2-Dibromoethene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.953 | ||
| EC Number |
| ||
| PubChem CID | |||
| UNII |
| ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C2H2Br2 | ||
| Molar mass | 185.846 g·mol | ||
| Appearance | colorless liquid | ||
| Density | 2.246 g/cm | ||
| Boiling point | 110 °C (230 °F; 383 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Toxic | ||
| GHS labelling: | |||
| Pictograms |   
| ||
| Signal word | Danger | ||
| Hazard statements | H301, H314, H315, H319, H335 | ||
| Precautionary statements | P260, P261, P264, P270, P271, P280, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
1,2-Dibromoethylene, also known as 1,2-dibromoethene and acetylene dibromide, is a dihalogenated unsaturated compound with one bromine on each of the two carbon atoms. There are two isomers of this compound, cis and trans. Both isomers are colorless liquids.
Synthesis
1,2-Dibromoethylene can be synthesized by halogenation of acetylene (C2H2) with bromine (Br2). In order to prevent the formation of tetrahalogenated compounds, acetylene is used in excess, with Br2 as the limiting reagent.
Alternately, halogenation of this kind could also be achieved through the use of two equivalents of N-bromosuccinimide and lithium bromide (LiBr). N-Bromosuccinimide provides Br as an electrophile, which is followed by Br from LiBr.
References
- "Chapter 9: Addition Reactions of Alkynes". Organic Chemistry 4e Carey. McGraw-Hill. Archived from the original on April 12, 2016. Retrieved 9 June 2017.
- Shao, L.-X.; Shi, M. (2006). "N-Bromosuccinimide and Lithium Bromide: An Efficient Combination for the Dibromination of Carbon–Carbon Unsaturated Bonds" (PDF). Synlett. 2006 (8): 1269–1271. doi:10.1055/s-2006-941558.