| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,2-Dichloroethene | |||
| Other names
1,2-Dichloroethene 1,2-DCE Acetylene dichloride sym-Dichloroethylene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.956 | ||
| EC Number |
| ||
| KEGG | |||
| PubChem CID | |||
| UNII |
| ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C2H2Cl2 | ||
| Molar mass | 96.95 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | sweet | ||
| Density | Z: 1.28 g/cm E: 1.26 g/cm | ||
| Melting point | Z: −81.47 °C E: −49.44 °C | ||
| Boiling point | Z: 60.2 °C E: 48.5 °C | ||
| Magnetic susceptibility (χ) |
| ||
| Dipole moment | Z: 1.9 D E: 0 D | ||
| Hazards | |||
| Flash point | 2–4 °C; 36–39 °F; 275–277 K | ||
| Explosive limits | 5.6–12.8% | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 770 mg/kg (oral, rat) 1275 mg/kg (oral, rat, trans-isomer) | ||
| LC50 (median concentration) | 21,273 ppm (mouse, 6 hr, trans-isomer) | ||
| LCLo (lowest published) | 16,000 ppm (rat, 6 hr, cis-isomer) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | TWA 200 ppm (790 mg/m) | ||
| REL (Recommended) | TWA 200 ppm (790 mg/m) | ||
| IDLH (Immediate danger) | 1000 ppm | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
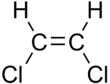
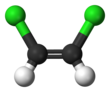
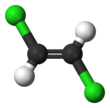
1,2-Dichloroethylene or 1,2-DCE is the name for a pair of organochlorine compounds with the molecular formula C2H2Cl2. The two compounds are isomers, each being colorless liquids with a sweet odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have some applications as a degreasing solvent. In contrast to most cis-trans compounds, the Z isomer (cis) is more stable than the E isomer (trans) by 0.4 kcal/mol.
Production, uses and reactions
cis-DCE, the Z isomer, is obtainable by the controlled chlorination of acetylene:
- C2H2 + Cl2 → C2H2Cl2
Industrially both isomers arise as byproducts of the production of vinyl chloride, which is produced on a vast scale. Unlike 1,1-dichloroethylene, the 1,2-dichloroethylene isomers do not polymerize.
trans-1,2-DCE has applications including electronics cleaning, precision cleaning, and certain metal cleaning applications.
Both isomers participate in Kumada coupling reactions. trans-1,2-Dichloroethylene participates in cycloaddition reactions.
Safety and environmental concerns
These compounds have "moderate oral toxicity to rats".
The dichloroethylene isomers occur in some polluted waters and soils, as the decomposition products of trichloroethylene. Significant attention has been paid to their further degradation, e.g. by iron particles.
See also
- 1,1-Dichloroethene
- 1,2-Dichloroethane, which is also often abbreviated as 1,2-DCE
References
- ^ E.-L. Dreher; T. R. Torkelson; K. K. Beutel (2011). "Chlorethanes and Chloroethylenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o06_o01. ISBN 978-3527306732.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0195". National Institute for Occupational Safety and Health (NIOSH).
- ^ "1,2-Dichloroethylene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Pitzer, Kenneth S.; Hollenberg, J. L. (1954). "cis- and trans-Dichloroethylenes. The Infrared Spectra from 130–400 Cm. and the Thermodynamic Properties". J. Am. Chem. Soc. 76 (6): 1493–1496. doi:10.1021/ja01635a010.
- "Chlorinated Solvents and Feed Stock - Axiall". Archived from the original on 2016-04-08. Retrieved 2016-03-23.
- Wang, Xiao Min; Hou, Xuelong; Zhou, Zhongyuan; Mak, Thomas C. W.; Wong, Henry N. C. (1993). "Arene synthesis by extrusion reaction. 16. Coplanar and stable derivatives of 13,14-didehydro-tribenzocyclooctene: Synthesis of 5,6-didehydro-1,1,14,14-tetramethyl-10,11-methano-1H-benzocyclooctafluorene and 5,6-didehydro-10,11-methano-1H-benzocyclooctafluorene-1,14-dione and x-ray crystal structures of 1,1,14,14-tetramethyl-10,11-methano-1H-benzofluorene and 1,12-dihydro-1,1,12,12-tetramethyldicyclopentatetraphenylene". The Journal of Organic Chemistry. 58 (26): 7498–7506. doi:10.1021/jo00078a031.
- Mattes, Timothy E.; Alexander, Anne K.; Coleman, Nicholas V. (2010). "Aerobic biodegradation of the chloroethenes: Pathways, enzymes, ecology, and evolution". FEMS Microbiology Reviews. 34 (4): 445–475. doi:10.1111/j.1574-6976.2010.00210.x. PMID 20146755.
- Schrick, Bettina; Blough, Jennifer L.; Jones, A. Daniel; Mallouk, Thomas E. (2002). "Hydrodechlorination of Trichloroethylene to Hydrocarbons Using Bimetallic Nickel−Iron Nanoparticles". Chemistry of Materials. 14 (12): 5140–5147. doi:10.1021/cm020737i.
External links
- International Chemical Safety Card 0436
- NIOSH Pocket Guide to Chemical Hazards. "#0195". National Institute for Occupational Safety and Health (NIOSH).