| This article needs attention from an expert in Chemistry. The specific problem is: Lots of missing key reagents in images and incorrect/confusing (possibly "non-chemist translation engine"?) chemical terminology. WikiProject Chemistry may be able to help recruit an expert. (September 2019) |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 1,3-Diphenyl-2-benzofuran | |
| Identifiers | |
| CAS Number | |
| ChemSpider | |
| ECHA InfoCard | 100.024.371 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | C20H14O |
| Molar mass | 270.33 g·mol |
| Appearance | pale yellow to dark yellow crystalline powder |
| Density | 1.0717 g·cm bei 25 °C |
| Melting point | * 125–126 °C
|
| Solubility in water | almost insoluble |
| Solubility in acetonitrile, benzene, dichloromethane, chloroform, dimethylsulfoxide, tetrahydrofuran or toluene | soluble |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
1,3-Diphenylisobenzofuran is a highly reactive diene that can scavenge unstable and short-lived dienophiles in a Diels-Alder reaction. It is furthermore used as a standard reagent for the determination of singlet oxygen, even in biological systems. Cycloadditions with 1,3-diphenylisobenzofuran and subsequent oxygen cleavage provide access to a variety of polyaromatics.
Preparation
The first synthesis of 1,3-diphenylisobenzofuran was reported in 1905 by A. Guyot and J. Catel. Phenylmagnesium bromide was reacted with 3-phenylphthalide (the latter accessible from the methyl ester of 3-hydroxyphthalide with phenylboronic acid in 95% yield) to a lactol, which gives with mineral acids upon elimination of water 1,3-diphenylisobenzofuran with 87% yield.

The patent literature describes the preparation of 1,3-diphenylisobenzofuran by cycloaddition of 1,3-butadiene and dibenzoylethylene (1,4-diphenyl-2-butene-1,4-dione, accessible from fumaryl chloride and benzene in the presence of aluminium chloride.). Dibenzoylethylene is predominantly present in the trans configuration but it can be converted into the needed cis configuration by simple heating.

The 4,5-dibenzoylcyclohexene formed previously is cyclized with acetic anhydride to the dihydroisobenzofuran. By bromine addition and hydrogen bromide elimination, 1,2-dibenzoylbenzene is formed and recyclized with zinc acetic acid to the final product 1,3-diphenylisobenzofuran. A publication from 1940 describes high yields for the individual stages of the extensive reaction sequence.
The (much cheaper) phthaloyl chloride gives also access to 1,2-dibenzoylbenzene via Friedel-Crafts acylation with benzene, which is reduced to 1,3-diphenylisobenzofuran in 78% yield using potassium borohydride.

The synthesis of 1,3-diarylisobenzofurans from 2-acylbenzaldehydes and boronic acids is less cumbersome and gives better yields,

just like the synthesis from salicylaldehydes via phenacylhydrazones, which undergo oxidation with lead(IV) acetate to give ortho-diketones, followed by the reaction with an aryl Grignard reagent.

Properties
1,3-Diphenylisobenzofuran is a yellow, light- and air-sensitive, crystalline solid that is soluble in many organic solvents with a maximum absorption around 420 nm (in solution), which generates intense fluorescence. Fluorescence measurements can be performed in DMF and DMSO because of the stability of 1,3-DPBF in those solvents. In chloroform and carbon tetrachloride the dissolved 1,3-diphenylisobenzofuran is rapidly photolyzed by attack of CHCl2 and CCl3 radicals, even in the absence of oxygen.
With ethanol, 1,3-diphenylisobenzofuran forms an orange-yellow, fluorescent solution. On irradiation, it forms a colorless photodimer (upon with exclusion of oxygen), upon discolouration of the solution.

The compound's refractive index is 1,6700 at 25 °C and 589 nm.
Use
Reagent for determination of singlet oxygen
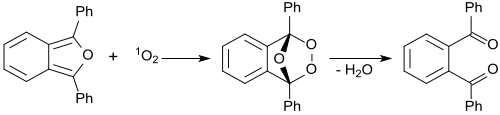


In the presence of methylene blue irradiated with red laser light, 1,3-diphenylisobenzofuran reacts with intermediate singlet oxygen O2, forming an unstable peroxide that decomposes into (colorless) 1,2-dibenzoylbenzene. The detection of singlet oxygen by 1,3-diphenylisobenzofuran is based on this reaction, even in biological systems. For biological systems, water-soluble derivatives of 1,3-diphenylisobenzofuran were developed. The singlet oxygen generation of photosensitizers were monitored by photolysis of 1,3-diphenylisobenzofuran (DPBF). 1,2-Dibenzoylbenzene absorbs at <300 nm, therefore making DPBF an optimal chemical trap for detecting singlet oxygen, as most photosensitizers absorb <400-600 nm. This allows for an accurate determination of the photodegradation of the molecule.
Dienophile in Diels-Alder reactions
Isobenzofurans like 1,3-diphenylisobenzofuran are among the most reactive Diels-Alder dienes known to date, and are useful for scavenging short-lived and unstable olefins and alkynes. The group led by Georg Wittig made important contributions to this topic.
With the unstable cyclohexyne, 1,3-diphenylisobenzofuran reacts to a tricyclic compound that gives a 9,10-diphenylcyclohexenonaphthalene after hydrogenation and hydrogen abstraction.

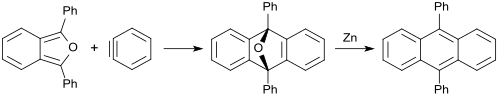
1,3-Diphenylisobenzofuran gives similarly with benzyne (dehydrobenzene) an oxygen-bridged anthracene (in 85% yield), which can be reduced with zinc to 9,10-diphenylanthracene (88% yield).
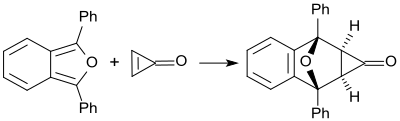
Cyclopropenone (which is unstable above its melting point of -29 °C) reacts quantitatively at room temperature with 1,3-diphenylisobenzofuran to form a Diels-Alder adduct, which is exclusively an exo isomer.
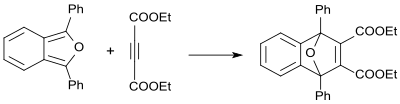
Dimethyl acetylenedicarboxylate reacts with 1,3-diphenylisobenzofuran as dienophile in 84% yield to yield the corresponding adduct.
1,3-Diphenylisobenzofuran reacts also with heterocyclic dienophiles such as 3-sulfolene to the corresponding Diels-Alder adduct.
Molecular building block for polyaromatics
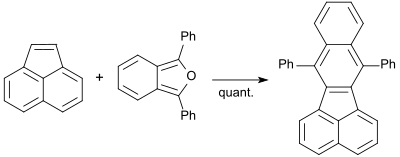
Polyaromatic hydrocarbons (PAHs) are of interest as precursors to graphite but also raise concern as ingredients of pollution. They have persistence and carcinogenicity. 1,3-diphenylisobenzofuran reacts quantitatively with acenaphthylene when heated to 160 °C to give 7,12-diphenylbenzofluoranthene.
The twice occurring Diels-Alder reaction of 1,3-diphenylisobenzofuran with p-benzoquinone yields almost quantitatively a product that can be reacted further with p-toluenesulfonic acid to give a pentacene derivative in 49% yield.

Literature
- W. Friedrichsen (1980), "Benzo[c]furans", Adv. Heterocycl. Chem., Advances in Heterocyclic Chemistry, vol. 26, pp. 135–234, doi:10.1016/S0065-2725(08)60141-5, ISBN 9780120206261
- W. Friedrichsen (1999), "Recent Advances in the Chemistry of Benzo[c]furans and Related Compounds", Adv. Heterocycl. Chem., Advances in Heterocyclic Chemistry, vol. 73, pp. 1–96, doi:10.1016/S0065-2725(08)60940-X, ISBN 9780120207732
- R. Rodrigo (1988), "Progress in the chemistry of isobenzofurans: Applications to the synthesis of natural products and polyaromatic hydrocarbons", Tetrahedron, vol. 44, no. 8, pp. 2093–2135, doi:10.1016/S0040-4020(01)81720-8
References
- "1,3-Diphenylisobenzofuran 5471-63-6 | TCI Deutschland GmbH". www.tcichemicals.com (in German). Retrieved 2018-01-14.
- ^ Sigma-Aldrich Co., product no. 105481.
- ^ Carl L. Yaws (2015), Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition, Oxford, UK: Elsevier Inc., p. 604, ISBN 978-0-323-28659-6
- ^ R. Adams; M.H. Gold (1940), "The Synthesis of 1,3-Diphenyldihydroisobenzofurans, 1,3-Diphenylisobenzofurans and o-Dibenzoylbenzenes from the Diene Addition Products to Dibenzoylethylene", Journal of the American Chemical Society, vol. 62, no. 1, pp. 56–61, Bibcode:1940JAChS..62...56A, doi:10.1021/ja01858a012
- P.C. Kierkus (2001), "1,3-Diphenylisobenzofuran", E-EROS Encyclopedia of Reagents for Organic Synthesis, doi:10.1002/047084289X.rd420, ISBN 0471936235
- R.H. Young; K. Wehrly; R.L. Martin (1971), "Solvent effects in dye-sensitized photooxidation reactions", Journal of the American Chemical Society, vol. 93, no. 22, pp. 5774–5779, Bibcode:1971JAChS..93.5774Y, doi:10.1021/ja00751a031
- J.A. Howard; G.D. Mendenhall (1975), "Autoxidation and photooxidation of 1,3-diphenylisobenzofuran: A kinetic and product study", Canadian Journal of Chemistry, vol. 53, no. 14, pp. 2199–2201, doi:10.1139/v75-307
- P. Carloni; et al. (1993), "On the use of 1,3-diphenylisobenzofuran (DPBF). Reactions with carbon and oxygen centered radicals in model and natural systems", Res. Chem. Intermed., vol. 19, no. 5, pp. 395–405, doi:10.1163/156856793X00181, S2CID 94802096
- A. Guyot, J. Catel, Bull. Soc. Chim. France, (35), 1124 (1906)
- A. Guyot, J. Catel, Compt. Rend. Hebd. Acad. Sci., Ser. C140, 1348 (1905)
- M. Kuriyama; N. Ishiyama; R. Shimazawa; R. Shirai; O. Onomura (2009), "Efficient synthesis of 3-arylphthalides using palladium-catalyzed arylation of aldehydes with organoboronic acids", Journal of Organic Chemistry, vol. 74, no. 23, pp. 9210–9213, doi:10.1021/jo901964k, PMID 19873994
- M. S. Newman (1961), "Evidence favoring a two-step mechanism for the Diels-Alder reaction", Journal of Organic Chemistry, vol. 26, no. 8, pp. 2630–2633, doi:10.1021/jo01066a004
- "trans-Dibenzoylethylene". Organic Syntheses. doi:10.15227/orgsyn.020.0029.
- US 2325727, R. Adams, "Dehydroisobenzofurans and process for preparing them", published 1943-08-03, assigned to E.I. du Pont de Nemours & Co.
- "1,2-Dibenzoylethylene, predominantly trans, 96%". Thermo Scientific Chemicals.
- D.V. Klemm; A. Tuncay (1989), "Photochemical and thermal isomerization of trans- and cis-1,2-dibenzoylethylene: A microscale approach", J. Chem. Educ., vol. 66, no. 6, p. 519, Bibcode:1989JChEd..66..519K, doi:10.1021/ed066p519
- US 2356907, R. Adams, "1,3-Diphenylisobenzofurans and process for preparing the same", published 1944-08-29, assigned to E.I. du Pont de Nemours & Co.
- Houben-Weyl Methods of Organic Chemistry (1973), Organometallic Compounds of Group II of the Periodic Table, 4th Edition, vol. XIII/2a, Stuttgart: Thieme, p. 419, ISBN 978-3-13-213204-7
- M. Cava; M.J. Mitchell; A.A. Deana (1960), "Condensed cyclobutane aromatic compounds. XIII. An attempted synthesis of 1,2-diphenylbenzocyclobutene", Journal of Organic Chemistry, vol. 25, no. 9, pp. 1481–1484, doi:10.1021/jo01079a005
- J. Jacq; B. Bessières; C. Einhorn; J. Einhorn (2010), "Regiospecific synthesis of functionalised 1,3-diarylisobenzofurans via palladium- and rhodium-catalysed reaction of boronic acids with o-acylbenzaldehydes under thermal or microwave activation", Org. Biomol. Chem., vol. 8, no. 21, pp. 4927–4933, doi:10.1039/c0ob00110d, PMID 20740250
- A. Kotali; P.G. Tsoungas (1987), "Oxidation of N-aroylhydrazones of o-hydroxyaryl ketones with lead(IV)acetate: A facile route to aromatic o-diketones", Tetrahedron Lett., vol. 28, no. 37, pp. 4321–4322, doi:10.1016/S0040-4039(00)96497-9
- J. Jacq; C. Einhorn; J. Einhorn (2008), "A versatile and regiospecific synthesis of functionalised 1,3-diarylisobenzofurans", Org. Lett., vol. 10, no. 17, pp. 3757–3760, doi:10.1021/ol801550a, PMID 18666776
- M. Wozniak; F. Tanfani; E. Bertoli; G. Zolese; J. Antonsiewicz (1991), "A new fluorescence method to detect singlet oxygen inside phospholipid model membranes", Biochim. Biophys. Acta, vol. 1082, no. 1, pp. 94–100, doi:10.1016/0005-2760(91)90304-Z, PMID 1849016
- X.-F. Zhang; X. Liu (2011), "The photostability and fluorescence properties of diphenylisobenzofuran", Journal of Luminiscence, vol. 131, no. 11, pp. 2263–2266, Bibcode:2011JLum..131.2263Z, doi:10.1016/j.jlumin.2011.05.048
- A. Schönberg; A. Mustafa; G. Aziz (September 1, 1954), "Diels-Alder Reaction. II. Experiments with 2-Styrylchromones. On the Nature of the Dimer of 1,3-Diphenylisobenzofuran", Journal of the American Chemical Society, vol. 76, no. 18, pp. 4576–4577, Bibcode:1954JAChS..76.4576S, doi:10.1021/ja01647a020
- Soman, Rahul; Raghav, Darpan; Sujatha, Subramaniam; Rathinasamy, Krishnan; Arunkumar, Chellaiah (30 Jun 2015). "Axial ligand modified high valent tin(iv) porphyrins: synthesis, structure, photophysical studies and photodynamic antimicrobial activities on Candida albicans". RSC Adv. 5 (75): 61103. Bibcode:2015RSCAd...561103S. doi:10.1039/C5RA09343K.
- "Grundpraktikum Physikalische Chemie, V28, Photooxidation von Diphenylisobenzofuran, Untersuchung der Reaktionskinetik durch Photometrie" (PDF) (in German). Universität Ulm. 2010-12-06. Archived from the original (PDF) on 2017-05-17. Retrieved 2017-08-30.
- C. Schmitz; J.M. Aubry; J. Rigaudy (1982), "A new access to the anthracene core: Synthesis of two water soluble singlet oxygen traps derived from 1,3-diphenylisobenzofuran and 9,10-diphenylanthracene", Tetrahedron, vol. 38, no. 10, pp. 1425–1430, doi:10.1016/0040-4020(82)80224-X
- D. Tobia; B. Rickborn (1987), "Substituent effects on rates of inter- and intramolecular cycloaddition reactions of isobenzofurans", Journal of Organic Chemistry, vol. 52, no. 12, pp. 2611–2615, doi:10.1021/jo00388a055
- G. Wittig (1963), "Über kleine Ringe mit Kohlenstoffdreifachbindung – noch eine Chemie des "Als ob"", Pure Appl. Chem. (in German), vol. 7, no. 2–3, pp. 173–192, doi:10.1351/pac196307020173, S2CID 95499778
- G. Wittig; E. Knauss; K. Niethamer (1960), "Über 9,10-Dihydroanthracen-Derivate mit Heterobrückenatomen", Justus Liebigs Ann. Chem. (in German), vol. 630, no. 1, pp. 10–18, doi:10.1002/jlac.19606300103
- R. Breslow; M. Oda (1972), "Isolation and characterization of pure cyclopropenone", Journal of the American Chemical Society, vol. 94, no. 13, pp. 4787–4788, Bibcode:1972JAChS..94.4787B, doi:10.1021/ja00768a089
- "Exo selectivity of the Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran". Retrieved 2017-08-28.
- J.A. Berson (1953), "Reactions of 1,3-diphenylisobenzofuran with acetylenic dienophiles", Journal of the American Chemical Society, vol. 75, no. 5, pp. 1240–1241, Bibcode:1953JAChS..75.1240B, doi:10.1021/ja01101a503
- M.P. Cava; J.P. VanMeter (1969), "Condensed cyclobutane aromatic compounds. XXX. Synthesis of some unusual 2,3-naphthoquinonoid heterocycles. A synthetic route to derivatives of naphthobiphenylene and anthracyclobutene", Journal of Organic Chemistry. Org. Chem., vol. 34, no. 3, pp. 538–545, doi:10.1021/jo01255a012
- Houben-Weyl Science of Synthesis (2009), Compounds with All-Carbon Functions, vol. 45b, Stuttgart: Thieme, p. 1038, ISBN 978-3-13-146551-1
- G.P. Miller; J. Briggs (2002), "Progress towards the synthesis of tris- and tetrakisfullerene adducts", Electrochem. Soc. Proc., vol. 2002–12, pp. 279–284, ISBN 1-56677-333-4