| |
| Names | |
|---|---|
| Preferred IUPAC name N,N′-Diphenylurea | |
| Other names 1,3-Diphenylurea; Diphenylurea; carbanilide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 782650 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.731 |
| EC Number |
|
| Gmelin Reference | 143821 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C13H12N2O |
| Molar mass | 212.252 g·mol |
| Melting point | 239–241 °C (462–466 °F; 512–514 K) |
| Boiling point | 262 °C (504 °F; 535 K) |
| Magnetic susceptibility (χ) | -127.5·10 cm/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H312, H332 |
| Precautionary statements | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
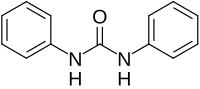
1,3-Diphenylurea is a phenylurea-type compound with the formula (PhNH)2CO (Ph = C6H5). It is a colorless solid that is prepared by transamidation of urea with aniline.
DPU is a cytokinin, a type of plant hormone that induces flower development. The cytokinin effect of DPU is relatively low, but other more potent phenylurea-type cytokinins have been reported.
It was detected in coconut milk.
References
- Effect of cytokinin-active phenylurea derivatives on shoot multiplication. T. Genkov and I. Ivanova, Bulg. J. Plant Physiol., 1995, 21(1), pages 73–83 (link to article at researchgate)
- Shantz, E. M.; Steward, F. C. (1955). "The Identification of Compound A from Coconut Milk as 1,3-Diphenylurea". Journal of the American Chemical Society. 77 (23): 6351. doi:10.1021/ja01628a079.
External links
 The dictionary definition of Diphenylurea at Wiktionary
The dictionary definition of Diphenylurea at Wiktionary
This botany article is a stub. You can help Misplaced Pages by expanding it. |
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |