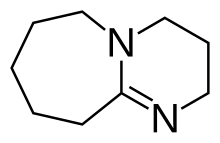
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 2,3,4,6,7,8,9,10-Octahydropyrimidoazepine | |
| Other names DBU, Diazabicycloundecene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.027.013 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H16N2 |
| Molar mass | 152.241 g·mol |
| Appearance | Colorless liquid |
| Density | 1.018 g/mL liquid |
| Melting point | −70 °C (−94 °F; 203 K) |
| Boiling point | 261 °C (502 °F; 534 K) (1 atm), 80 to 83 °C (0.6 mmHg) |
| Solubility in water | ethers, alcohols |
| Acidity (pKa) | 13.5±1.5 (pKa of conjugate acid in water); 24.34 (pKa of conjugate acid in acetonitrile) |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H301, H302, H312, H314, H412 |
| Precautionary statements | P260, P264, P270, P273, P280, P301+P310, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P405, P501 |
| Flash point | 119.9 °C (247.8 °F; 393.0 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
1,8-Diazabicycloundec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst, a complexing ligand, and a non-nucleophilic base.
Occurrence
Although all commercially available DBU is produced synthetically, it may also be isolated from the sea sponge Niphates digitalis. The biosynthesis of DBU has been proposed to begin with adipaldehyde and 1,3-diaminopropane.

Uses
As a reagent in organic chemistry, DBU is used as a ligand and base. As a base, protonation occurs at the imine nitrogen. Lewis acids also attach to the same nitrogen.
These properties recommend DBU for use as a catalyst, for example as a curing agent for epoxy resins and polyurethane.
It is used in the separation of fullerenes in conjunction with trimethylbenzene. It reacts with C70 and higher fullerenes, but not with C60.
It is useful for dehydrohalogenations.
See also
References
- Kaupmees, K.; Trummal, A.; Leito, I. (2014). "Basicities of Strong Bases in Water: A Computational Study". Croat. Chem. Acta. 87 (4): 385–395. doi:10.5562/cca2472.
- Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". J. Org. Chem. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- Ghosh, Nandita (2004). "DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) - A Nucleophillic Base". Synlett (3): 574–575. doi:10.1055/s-2004-815436.
- ^ E. L. Regalado; Judith Mendiola; Abilio Laguna; Clara Nogueiras; Olivier P Thomas (2010). "Polar alkaloids from the Caribbean marine sponge Niphates digitalis". Nat. Prod. Commun. 5 (8): 1187–1190. PMID 20839615.
- Parviainen, Arno; King, Alistair W. T.; Mutikainen, Ilpo; Hummel, Michael; Selg, Christoph; Hauru, Lauri K. J.; Sixta, Herbert; Kilpeläinen, Ilkka (2013). "Predicting Cellulose Solvating Capabilities of Acid–Base Conjugate Ionic Liquids". ChemSusChem. 6 (11): 2161–2169. Bibcode:2013ChSCh...6.2161P. doi:10.1002/cssc.201300143. ISSN 1864-5631. PMID 24106149.
- Romero, Erik A.; Zhao, Tianxiang; Nakano, Ryo; Hu, Xingbang; Wu, Youting; Jazzar, Rodolphe; Bertrand, Guy (2018-10-01). "Tandem copper hydride–Lewis pair catalysed reduction of carbon dioxide into formate with dihydrogen". Nature Catalysis. 1 (10): 743–747. doi:10.1038/s41929-018-0140-3. ISSN 2520-1158.
- Savoca, Ann C.; Urgaonkar, Sameer (2006). "1,8-Diazabicyclo[5.4.0]undec-7-ene". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd011.pub2. ISBN 0-471-93623-5.