| |
| |

| |
| Names | |
|---|---|
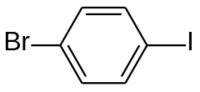
| Preferred IUPAC name 1-Bromo-4-iodobenzene | |
| Other names p-Bromoiodobenzene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.008.785 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H4BrI |
| Molar mass | 282.90 g/mol |
| Appearance | colorless solid |
| Melting point | 91 °C (196 °F; 364 K) |
| Related compounds | |
| Related compounds | 1,4-Dibromobenzene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
1-Bromo-4-iodobenzene is a mixed aryl halide (aryl bromide and aryl iodide) with the formula BrC6H4I.
Preparation
In one laboratory route to 1-bromo-4-iodobenzene, 4-bromoaniline is treated with concentrated sulfuric acid and sodium nitrite to form the diazonium salt, which is then treated with potassium iodide to form 1-bromo-4-iodobenzene.
Reactions
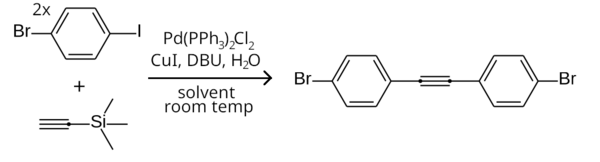
Since aryl iodides are more reactive than aryl bromides in the Sonogashira coupling, the iodine end of 1-bromo-4-iodobenzene can be selectively coupled to a terminal acetylene while leaving the bromine end unreacted, by running the reaction at room temperature. For example, two equivalents of 1-bromo-4-iodobenzene can couple to trimethylsilylacetylene in a room temperature symmetrical Sonogashira coupling (with TMSA being deprotected to acetylene in-situ) to form bis(4-bromophenyl)acetylene.
See also
References
- "1-Bromo-4-iodobenzene". ChemSpider. Retrieved 21 October 2023.
- PubChem. "1-Bromo-4-iodobenzene". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-10-22.
- Banerjee, M.; Shukla, R.; Rathore, R. (15 January 2009). "Synthesis, Optical, and Electronic Properties of Soluble Poly-p-phenylene Oligomers as Models for Molecular Wires". Journal of the American Chemical Society. 131 (5): 1780–1786. doi:10.1021/ja805102d. PMID 19146375.
- Chinchilla, R.; Nájera, C. (2007), "The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry", Chem. Rev., 107 (3): 874–922, doi:10.1021/cr050992x, PMID 17305399
- Mio, Matthew J.; Kopel, Lucas C.; Braun, Julia B.; Gadzikwa, Tendai L.; Hull, Kami L.; Brisbois, Ronald G.; Markworth, Christopher J.; Grieco, Paul A. (2002). "One-Pot Synthesis of Symmetrical and Unsymmetrical Bisarylethynes by a Modification of the Sonogashira Coupling Reaction". Organic Letters. 4 (19): 3199–3202. doi:10.1021/ol026266n. PMID 12227748.