| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2,4,6-Tribromophenol | |||
| Other names Tribromophenol; 2,4,6-TBP; TBP | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.890 | ||
| KEGG | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H3Br3O | ||
| Molar mass | 330.801 g·mol | ||
| Appearance | White needles or prisms | ||
| Melting point | 95.5 °C (203.9 °F; 368.6 K) | ||
| Boiling point | 244 °C (471 °F; 517 K) 286 °C | ||
| Solubility in water | Slightly soluble 59-61 mg/L | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |  
| ||
| NFPA 704 (fire diamond) |
 | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 2000 mg/kg (rat, oral) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
2,4,6-Tribromophenol (TBP) is a brominated derivative of phenol. It is used as a fungicide, as a wood preservative, and an intermediate in the preparation of flame retardants.
Production
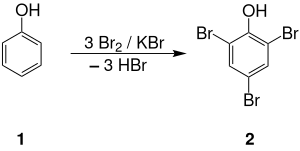
Although natural TBP has been identified in ocean sediments as a metabolite of marine fauna, the commercial product is prepared industrially. In 2001, the production volume of TBP was estimated to be 2500 tonnes/year in Japan and 9500 tonnes/year worldwide. TBP can be prepared by the controlled reaction of elemental bromine with phenol:
Uses
The predominant use of TBP is as an intermediate in the preparation of flame retardants such as brominated epoxy resins. TBP is reacted with sodium hydroxide to form the sodium salt, which is used as a fungicide and wood preservative.
Bismuth salt
The bismuth salt is the active ingredient in Xeroform dressing.
Metabolism
Microbial metabolism in products treated with TBP is known to produce 2,4,6-tribromoanisole (TBA), which has a musty odor. In 2010 and 2011, Pfizer and Johnson & Johnson voluntarily recalled some products due to TBA odors from wooden pallets which were treated with TBP.
References
- ^ "3851: Tribromophenol" in Gardner's Commercially Important Chemicals: Synonyms, Trade Names, and Properties, G. W. A. Milne (Editor), ISBN 978-0-471-73518-2, page 632
- ^ Concise International Chemical Assessment Document 66: 2,4,6-Tribromophenol and Other Simple Brominated Phenols, International Programme on Chemical Safety
- ^ Merck Index, 11th Edition, 9526
- Sigma-Aldrich Co., 2,4,6-Tribromophenol. Retrieved on 2015-02-19.
- Fielman KT, Woodin SA, Lincoln DE (2001). "Polychaete indicator species as a source of natural halogenated organic compounds in marine sediments". Environmental Toxicology and Chemistry. 20 (4): 738–747. doi:10.1002/etc.5620200407. PMID 11345448. S2CID 42911887.
- "2,4,6 Tribromophenol" (PDF). ICL Industrial Products. Archived from the original (PDF) on August 27, 2019. Retrieved April 13, 2022.
- Tsunoda K, Takahashi M (1989). "Laboratory Evaluation of Chemicals as Wood Prerservatives: (1) Tribromophenol" (PDF). Wood Research. 76. Kyoto University: 39–48.
- "MeSH Browser". meshb.nlm.nih.gov. Retrieved 2019-08-27.
- Frank B. Whitfield, Jodie L. Hill, Kevin J. Shaw (1997). "2,4,6-Tribromoanisole: a Potential Cause of Mustiness in Packaged Food". J. Agric. Food Chem. 45 (3): 889–893. doi:10.1021/jf960587u.
- 38,000 more bottles of Lipitor recalled over odor complaints, CNN.com, October 30, 2010
- Lipitor (atorvastatin) 40 mg: Recall Specific Bottles, drugs.com, Dec 23, 2010
- Tylenol Recall Expands, WebMD Health News, January 18, 2010
- McNeil Consumer Healthcare Announces Voluntary Recall Of One Product Lot Of TYLENOL Extra Strength Caplets 225 Count Distributed In The U.S.