
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 2-Methyl-1H-imidazole | |
| Other names 2-MeIm | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.010.697 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H6N2 |
| Molar mass | 82.10 g/mol |
| Appearance | white or colorless solid |
| Melting point | 145 °C (293 °F; 418 K) |
| Boiling point | 270 °C (518 °F; 543 K) |
| Solubility in water | 0.29 g/ml |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | causes skin rashes and eye irritation |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
2-Methylimidazole is an organic compound that is structurally related to imidazole with the chemical formula CH3C3H2N2H. It is a white or colorless solid that is highly soluble in polar organic solvents and water. It is a precursor to a range of drugs and is a ligand in coordination chemistry.
Synthesis and reactions
It is prepared by condensation of glyoxal, ammonia and acetaldehyde, a Radziszewski reaction. Nitration gives 5-nitro derivative.
2-Methylimidazole is a sterically hindered imidazole that is used to simulate the coordination of histidine to heme complexes. It can be deprotonated to make imidazolate-based coordination polymers.
Applications
2-Methylimidazole is a precursor to the several members of the nitroimidazole antibiotics that are used to combat anaerobic bacterial and parasitic infections.
- Nitroimidazole antibiotics and antiprotozoals containing 2-methylimidazole cores:
-
 Dimetridazole
Dimetridazole
-
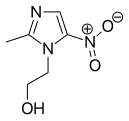 Metronidazole
Metronidazole
-
 Secnidazole
Secnidazole
-
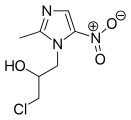 Ornidazole
Ornidazole
-
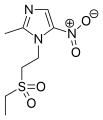 Tinidazole
Tinidazole
-
 Carnidazole
Carnidazole
Safety
It has low toxicity with an LD50 (rat, oral) of 1300 mg/kg, but it is strongly irritating to the skin and eyes.
2-Methylimidazole is a REACH Regulation Candidate Substance of Very High Concern due to its endocrine disrupting properties.
References
- ^ Ebel, K., Koehler, H., Gamer, A. O., & Jäckh, R. "Imidazole and Derivatives." In Ullmann’s Encyclopedia of Industrial Chemistry; 2002 Wiley-VCH, doi:10.1002/14356007.a13_661
- Banerjee, Rahul; Phan, Anh; Wang, Bo; Knobler, Carolyn; Furukawa, Hiroyasu; O'Keeffe, Michael; Yaghi, Omar M (2008). "High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture". Science. 319 (5865): 939–943. doi:10.1126/science.1152516. PMID 18276887. S2CID 22210227.
- Edwards, David I (1993). "Nitroimidazole drugs - action and resistance mechanisms. I. Mechanism of action". Journal of Antimicrobial Chemotherapy. 31 (1): 9–20. doi:10.1093/jac/31.1.9. PMID 8444678.
- Schilliger-Musset, Christel (2020-06-18). "D(2020)4578-DC, "Inclusion of substances of very high concern in the Candidate List for eventual inclusion in Annex XIV (Decision of the European Chemicals Agency)"". European Chemicals Agency. Archived from the original on 2020-06-30.